Broad Host Tropism of Flaviviruses during the Entry Stage
- PMID: 36943072
- PMCID: PMC10101140
- DOI: 10.1128/spectrum.05281-22
Broad Host Tropism of Flaviviruses during the Entry Stage
Abstract
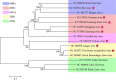
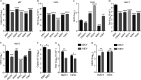
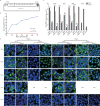
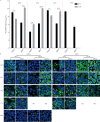
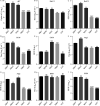
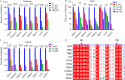
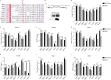
The genus Flavivirus consists of viruses with various hosts, including insect-specific flaviviruses (ISFs), mosquito-borne flaviviruses (MBFs), tick-borne flaviviruses (TBFs), and no-known vector (NKV) flaviviruses. Using the reporter viral particle (RVP) system, we found the efficient entry of ISFs into vertebrate cells, MBFs into tick cells, as well as NKVs and TBFs into mosquito cells with similar entry characteristics. By construction of reverse genetics, we found that Yokose virus (YOKV), an NKV, could enter and replicate in mosquito cells but failed to produce infectious particles. The complete removal of the glycosylation modification on the envelope proteins of flaviviruses had no obvious effect on the entry of all MBFs and TBFs. Our results demonstrate an entry-independent host-tropism mechanism and provide a new insight into the evolution of flaviviruses. IMPORTANCE Vector-borne flaviviruses, such as Zika virus, have extremely broad host and cell tropism, even though no critical entry receptors have yet been identified. Using an RVP system, we found the efficient entry of ISFs, MBFs, TBFs, and NKVs into their nonhost cells with similar characteristics. However, glycan-binding proteins cannot serve as universal entry receptors. Our results demonstrate an entry-independent host-tropism mechanism and give a new insight into the cross-species evolution of flaviviruses.
Keywords: Zika virus; entry independent; flaviviruses; host tropism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Guzman H, Contreras-Gutierrez MA, Travassos da Rosa APA, Nunes MRT, Cardoso JF, Popov VL, Young KI, Savit C, Wood TG, Widen SG, Watts DM, Hanley KA, Perera D, Fish D, Vasilakis N, Tesh RB. 2018. Characterization of three new insect-specific flaviviruses: their relationship to the mosquito-borne flavivirus pathogens. Am J Trop Med Hyg 98:410–419. doi: 10.4269/ajtmh.17-0350. - DOI - PMC - PubMed
-
- Lindenbach BD, Thiel H-J, Rice CM. 2007. Flaviviruses: the viruses and their replication, p 1101–1152. In Knipe DM, Howley PM (ed), Fields' virologv, 5th ed. Lippincott, Williams and Wilkins, Philadelphia, PA.
LinkOut - more resources
Full Text Sources

