P22-Based Nanovaccines against Enterohemorrhagic Escherichia coli
- PMID: 36943089
- PMCID: PMC10100862
- DOI: 10.1128/spectrum.04734-22
P22-Based Nanovaccines against Enterohemorrhagic Escherichia coli
Abstract
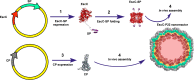
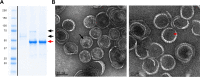
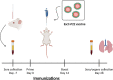
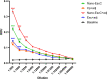
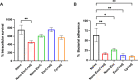
Enterohemorrhagic Escherichia coli (EHEC) is an important causative agent of diarrhea in humans that causes outbreaks worldwide. Efforts have been made to mitigate the morbidity and mortality caused by these microorganisms; however, the global incidence is still high, causing hundreds of deaths per year. Several vaccine candidates have been evaluated that demonstrate some stability and therapeutic potential but have limited overarching effect. Virus-like particles have been used successfully as nanocontainers for the targeted delivery of drugs, proteins, or nucleic acids. In this study, phage P22 nanocontainers were used as a carrier for the highly antigenic T3SS structural protein EscC that is conserved between EHEC and other enteropathogenic bacteria. We were able to stably incorporate the EscC protein into P22 nanocontainers. The EscC-P22 particles were used to intranasally inoculate mice, which generated specific antibodies against EscC. These antibodies increased the phagocytic activity of murine macrophages infected with EHEC in vitro and reduced bacterial adherence to Caco-2 epithelial cells in vitro, illustrating their functionality. The EscC-P22-based particles are a potential nanovaccine candidate for immunization against EHEC O157:H7 infections. IMPORTANCE This study describes the initial attempt to use P22 viral-like particles as nanocontainers expressing enterohemorrhagic Escherichia coli (EHEC) proteins that are immunogenic and could be used as effective vaccines against EHEC infections.
Keywords: EHEC; P22-based nanovaccines; nanocontainer; pathogenic Escherichia coli.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Development of a Gold Nanoparticle Vaccine against Enterohemorrhagic Escherichia coli O157:H7.mBio. 2019 Aug 13;10(4):e01869-19. doi: 10.1128/mBio.01869-19. mBio. 2019. PMID: 31409688 Free PMC article.
-
Optimization of Multivalent Gold Nanoparticle Vaccines Eliciting Humoral and Cellular Immunity in an In Vivo Model of Enterohemorrhagic Escherichia coli O157:H7 Colonization.mSphere. 2022 Feb 23;7(1):e0093421. doi: 10.1128/msphere.00934-21. Epub 2022 Jan 19. mSphere. 2022. PMID: 35044806 Free PMC article.
-
Comparative genomics and immunoinformatics approach for the identification of vaccine candidates for enterohemorrhagic Escherichia coli O157:H7.Infect Immun. 2014 May;82(5):2016-26. doi: 10.1128/IAI.01437-13. Epub 2014 Mar 4. Infect Immun. 2014. PMID: 24595137 Free PMC article.
-
[Enterohemorrhagic Escherichia coli and hemolytic-uremic syndrome].Wien Klin Wochenschr. 1997 Sep 19;109(17):669-77. Wien Klin Wochenschr. 1997. PMID: 9381722 Review. German.
-
Interkingdom Chemical Signaling in Enterohemorrhagic Escherichia coli O157:H7.Adv Exp Med Biol. 2016;874:201-13. doi: 10.1007/978-3-319-20215-0_9. Adv Exp Med Biol. 2016. PMID: 26589220 Review.
Cited by
-
Decoration of Burkholderia Hcp1 protein to virus-like particles as a vaccine delivery platform.Infect Immun. 2024 Mar 12;92(3):e0001924. doi: 10.1128/iai.00019-24. Epub 2024 Feb 14. Infect Immun. 2024. PMID: 38353543 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

