Biosynthesis of 3,6-Dideoxy-heptoses for the Capsular Polysaccharides of Campylobacter jejuni
- PMID: 36943186
- PMCID: PMC10440746
- DOI: 10.1021/acs.biochem.3c00012
Biosynthesis of 3,6-Dideoxy-heptoses for the Capsular Polysaccharides of Campylobacter jejuni
Abstract
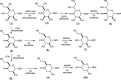
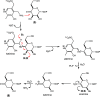
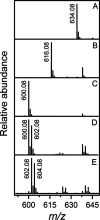
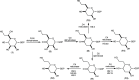
Campylobacter jejuni is the leading cause of food poisoning in the United States. Surrounding the exterior surface of this bacterium is a capsular polysaccharide (CPS) that helps protect the organism from the host immune system. The CPS is composed of a repeating sequence of common and unusual sugar residues, including relatively rare heptoses. In the HS:5 serotype, we identified four enzymes required for the biosynthesis of GDP-3,6-dideoxy-β-l-ribo-heptose. In the first step, GDP-d-glycero-α-d-manno-heptose is dehydrated to form GDP-6-deoxy-4-keto-α-d-lyxo-heptose. This product is then dehydrated by a pyridoxal phosphate-dependent C3-dehydratase to form GDP-3,6-dideoxy-4-keto-α-d-threo-heptose before being epimerized at C5 to generate GDP-3,6-dideoxy-4-keto-β-l-erythro-heptose. In the final step, a C4-reductase uses NADPH to convert this product to GDP-3,6-dideoxy-β-l-ribo-heptose. These results are at variance with the previous report of 3,6-dideoxy-d-ribo-heptose in the CPS from serotype HS:5 of C. jejuni. We also demonstrated that GDP-3,6-dideoxy-β-l-xylo-heptose is formed using the corresponding enzymes found in the gene cluster from serotype HS:11 of C. jejuni. The utilization of different C4-reductases from other serotypes of C. jejuni enabled the formation of GDP-3,6-dideoxy-α-d-arabino-heptose and GDP-3,6-dideoxy-α-d-lyxo-heptose.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Monteiro M. A.; Noll A.; Laird R. M.; Pequegnat B.; Ma Z. C.; Bertolo L.; DePass C.; Omari E.; Gabryelski P.; Redkyna O.; Jiao Y. N.; Borrelli S.; Poly F.; Guerry P.. Campylobacter jejuniCapsule Polysaccharide Conjugate Vaccine. In Carbohydrate-based Vaccines: from Concept to Clinic; American Chemical Society: Washington, DC, 2018, 249–271.
-
- Michael F.; Szymanski C. M.; Li J. J.; Chan K. H.; Khieu N. H.; Larocque S.; Wakarchuk W. W.; Brisson J. R.; Monteiro M. A. The Structures of the Lipooligosaccharide and Capsule Polysaccharide of Campylobacter jejuni Genome Sequenced Strain NCTC 11168. Eur. J. Biochem. 2002, 269, 5119–5136. 10.1046/j.1432-1033.2002.03201.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

