Human PIK3R1 mutations disrupt lymphocyte differentiation to cause activated PI3Kδ syndrome 2
- PMID: 36943234
- PMCID: PMC10037341
- DOI: 10.1084/jem.20221020
Human PIK3R1 mutations disrupt lymphocyte differentiation to cause activated PI3Kδ syndrome 2
Abstract
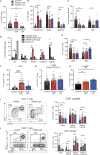
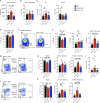
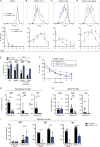
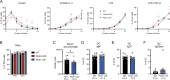
Heterozygous loss-of-function (LOF) mutations in PIK3R1 (encoding phosphatidylinositol 3-kinase [PI3K] regulatory subunits) cause activated PI3Kδ syndrome 2 (APDS2), which has a similar clinical profile to APDS1, caused by heterozygous gain-of-function (GOF) mutations in PIK3CD (encoding the PI3K p110δ catalytic subunit). While several studies have established how PIK3CD GOF leads to immune dysregulation, less is known about how PIK3R1 LOF mutations alter cellular function. By studying a novel CRISPR/Cas9 mouse model and patients' immune cells, we determined how PIK3R1 LOF alters cellular function. We observed some overlap in cellular defects in APDS1 and APDS2, including decreased intrinsic B cell class switching and defective Tfh cell function. However, we also identified unique APDS2 phenotypes including defective expansion and affinity maturation of Pik3r1 LOF B cells following immunization, and decreased survival of Pik3r1 LOF pups. Further, we observed clear differences in the way Pik3r1 LOF and Pik3cd GOF altered signaling. Together these results demonstrate crucial differences between these two genetic etiologies.
© 2023 Nguyen et al.
Conflict of interest statement
Disclosures: A. Lau reported being an employee of AstraZeneca since January 2023. J. Bier reported being an employee of Takeda Pharmaceutical since September 2022. R.S. Abraham reported personal fees from Enzyvant Therapeutics, Horizon Pharma, Beckman Coulter, and ClinGen outside the submitted work. C. Burkhart reported being an employee and shareholder of Novartis Pharma AG. Leniolisib is a p110δ-specific inhibitor and was discovered and developed by Novartis Pharma AG. Leniolisib was provided in 2016 to S.G. Tangye for studies described in the manuscript. Leniolisib was outlicensed to Pharming Group N.V. in 2019. S.G. Tangye reported being on the Pharming Group NV Global Advisory Board for the use of leniolisib to treat individuals with inborn errors of immunity due to mutations in PIK3CD or PIK3R1. Pharming has licensed leniolisib from Novartis as a therapy for these conditions. E.K. Deenick reported “other” from CSL outside the submitted work. No other disclosures were reported.
Figures


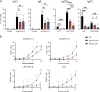


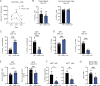
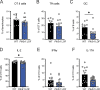
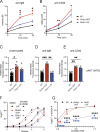
References
-
- Asano, T., Okada S., Tsumura M., Yeh T.W., Mitsui-Sekinaka K., Tsujita Y., Ichinose Y., Shimada A., Hashimoto K., Wada T., et al. . 2018. Enhanced AKT phosphorylation of circulating B cells in patients with activated PI3Kδ syndrome. Front. Immunol. 9:568. 10.3389/fimmu.2018.00568 - DOI - PMC - PubMed
-
- Avery, D.T., Deenick E.K., Ma C.S., Suryani S., Simpson N., Chew G.Y., Chan T.D., Palendira U., Bustamante J., Boisson-Dupuis S., et al. . 2010. B cell-intrinsic signaling through IL-21 receptor and STAT3 is required for establishing long-lived antibody responses in humans. J. Exp. Med. 207:155–171. 10.1084/jem.20091706 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

