Inactivating the lipid kinase activity of PI3KC2β is sufficient to rescue myotubular myopathy in mice
- PMID: 36943412
- PMCID: PMC10243799
- DOI: 10.1172/jci.insight.151933
Inactivating the lipid kinase activity of PI3KC2β is sufficient to rescue myotubular myopathy in mice
Abstract
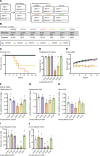
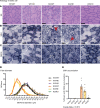
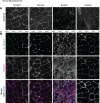
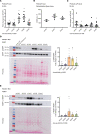
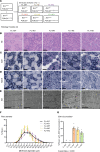
Phosphoinositides (PIs) are membrane lipids that regulate signal transduction and vesicular trafficking. X-linked centronuclear myopathy (XLCNM), also called myotubular myopathy, results from loss-of-function mutations in the MTM1 gene, which encodes the myotubularin phosphatidylinositol 3-phosphate (PtdIns3P) lipid phosphatase. No therapy for this disease is currently available. Previous studies showed that loss of expression of the class II phosphoinositide 3-kinase (PI3K) PI3KC2β (PI3KC2B) protein improved the phenotypes of an XLCNM mouse model. PI3Ks are well known to have extensive scaffolding functions and the importance of the catalytic activity of this PI3K for rescue remains unclear. Here, using PI3KC2β kinase-dead mice, we show that the selective inactivation of PI3KC2β kinase activity is sufficient to fully prevent muscle atrophy and weakness, histopathology, and sarcomere and triad disorganization in Mtm1-knockout mice. This rescue correlates with normalization of PtdIns3P level and mTORC1 activity, a key regulator of protein synthesis and autophagy. Conversely, lack of PI3KC2β kinase activity did not rescue the histopathology of the BIN1 autosomal CNM mouse model. Overall, these findings support the development of specific PI3KC2β kinase inhibitors to cure myotubular myopathy.
Keywords: Inositol phosphates; Muscle Biology; Protein kinases; Skeletal muscle.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

