NHA1 is a cation/proton antiporter essential for the water-conserving functions of the rectal complex in Tribolium castaneum
- PMID: 36943876
- PMCID: PMC10068851
- DOI: 10.1073/pnas.2217084120
NHA1 is a cation/proton antiporter essential for the water-conserving functions of the rectal complex in Tribolium castaneum
Abstract
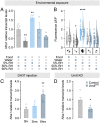
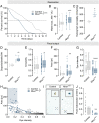
More than half of all extant metazoan species on earth are insects. The evolutionary success of insects is linked with their ability to osmoregulate, suggesting that they have evolved unique physiological mechanisms to maintain water balance. In beetles (Coleoptera)-the largest group of insects-a specialized rectal ("cryptonephridial") complex has evolved that recovers water from the rectum destined for excretion and recycles it back to the body. However, the molecular mechanisms underpinning the remarkable water-conserving functions of this system are unknown. Here, we introduce a transcriptomic resource, BeetleAtlas.org, for the exceptionally desiccation-tolerant red flour beetle Tribolium castaneum, and demonstrate its utility by identifying a cation/H+ antiporter (NHA1) that is enriched and functionally significant in the Tribolium rectal complex. NHA1 localizes exclusively to a specialized cell type, the leptophragmata, in the distal region of the Malpighian tubules associated with the rectal complex. Computational modeling and electrophysiological characterization in Xenopus oocytes show that NHA1 acts as an electroneutral K+/H+ antiporter. Furthermore, genetic silencing of Nha1 dramatically increases excretory water loss and reduces organismal survival during desiccation stress, implying that NHA1 activity is essential for maintaining systemic water balance. Finally, we show that Tiptop, a conserved transcription factor, regulates NHA1 expression in leptophragmata and controls leptophragmata maturation, illuminating the developmental mechanism that establishes the functions of this cell. Together, our work provides insights into the molecular architecture underpinning the function of one of the most powerful water-conserving mechanisms in nature, the beetle rectal complex.
Keywords: Tribolium castaneum; cation/H+ antiporter; genetics; rectal complex; secondary cell.
Conflict of interest statement
The authors declare no competing interest.
Figures





References
-
- Eggleton P., The state of the world’s insects. Annu. Rev. Environ. Res. 45, 61–82 (2020).
-
- Dow J. A. T., Halberg K. A., Terhzaz S., Davies S. A., “Drosophila as a model for neuroendocrine control of renal homeostasis“ in Model Animals in Neuroendocrinology: From Worms to Mouse to Man, M. Ludwig & G. Levkowitz, Eds. (Wiley-Blackwell, 2018), Vol. 1, pp. 81–100.
-
- Maddrell S. H. P., The functional design of the insect excretory system. J. Exp. Biol. 90, 1–15 (1981).
-
- Phillips J. E., Hanrahan J., Chamberlin M., Thomson B., “Mechanisms and control of reabsorption in insect hindgut“ in Advances in Insect Physiology, Evans P. D., Wigglesworth V. B., Eds. (Academic Press, 1987), vol. 19, pp. 329–422.
-
- J. Machin, Water balance inTenebrio molitor, L, Larvae; the effect of atmospheric water absorption. J. Comparat. Physiol. 101, 121–132 (1975).

