Transcatheter Aortic Valve Replacement in Large Annuli Valves With the Supra-Annular, Self-Expandable Evolut Platform in a Real-World Registry
- PMID: 36943929
- PMCID: PMC10022658
- DOI: 10.1161/CIRCINTERVENTIONS.122.012623
Transcatheter Aortic Valve Replacement in Large Annuli Valves With the Supra-Annular, Self-Expandable Evolut Platform in a Real-World Registry
Abstract
Background: Transcatheter aortic valve replacement is approved for treatment of patients with severe aortic stenosis across the spectrum of risk. While considering broader indications for use, transcatheter aortic valve replacement in large native annuli has become increasingly important.
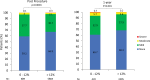
Methods: Patients with tricuspid aortic stenosis undergoing transcatheter aortic valve replacement using the Evolut R or Evolut PRO+ 34 mm valves (Medtronic, Minneapolis, MN) in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry between October 2016 and September 2020 were stratified according to in range (>12%) device oversizing and below range (0%-12%) device oversizing. Patients undergoing valve-in-valve procedures, having a baseline annulus size <26 or ≥34 mm, or without computed tomography angiography measured annulus size were excluded. Percentage of oversizing was calculated as [(valve diameter-annulus diameter)×100/annulus diameter].
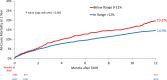
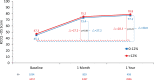
Results: Transcatheter aortic valve replacement in patients with large annuli was performed in 8017 patients with a mean (±SD) age 79.3±7.9 years and 94% were male. Below range (n=1096) was less common than in range oversizing (n=6921). At 1-year follow-up, mortality (19.6% versus 14.9%; P=0.001), aortic valve reintervention (2.1% versus 0.6%; P<0.001) and valve-related readmission rates (3.2% versus 2.0%; P=0.014) were higher in the below range device oversizing group versus in range group respectively. In a multivariable Cox proportional hazards regression model, when controlling for clinically relevant covariates, below range device oversizing was associated with higher 1-year all-cause mortality (HR, 1.28 [CI, 1.07-1.51]; P=0.005).
Conclusions: Results from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry in patients with large annuli valves using 34mm Evolut R/PRO+ valves suggest that in range (>12%) device oversizing delivered better clinical outcomes than implantation with below range (0%-12%) device oversizing.
Keywords: aortic valve stenosis; bioprosthesis; transcatheter aortic valve replacement.
Conflict of interest statement
Dr Tang is a physician proctor with Medtronic and a consultant for Medtronic, Abbott Structural Heart and W. L. Gore & Associates. Dr Forrest is a consultant for Medtronic and Edwards Lifesciences and receives grant support from Medtronic and Edwards Lifesciences. Dr Reardon is a consultant for Medtronic, W.L. Gore and Associates and Boston Scientific. Dr Szeto is an investigator and on the advisory board of Medtronic and Edwards Lifesciences. Dr Kodali has received grants and research support from Medtronic, Boston Scientific, Claret Medical, and Edwards Lifesciences. R. Eisenberg is an employee and shareholder of Medtronic. Dr Attizzani is a consultant and serves as a proctor and is on the advisory board of Medtronic and is a consultant for Abbott Vascular.
Figures

Similar articles
-
Bioprosthetic Valve Performance After Transcatheter Aortic Valve Replacement With Self-Expanding Versus Balloon-Expandable Valves in Large Versus Small Aortic Valve Annuli: Insights From the CHOICE Trial and the CHOICE-Extend Registry.JACC Cardiovasc Interv. 2018 Dec 24;11(24):2507-2518. doi: 10.1016/j.jcin.2018.07.050. Epub 2018 Nov 28. JACC Cardiovasc Interv. 2018. PMID: 30503595 Clinical Trial.
-
Transcatheter Self-Expandable Valve Implantation for Aortic Stenosis in Small Aortic Annuli: The TAVI-SMALL Registry.JACC Cardiovasc Interv. 2020 Jan 27;13(2):196-206. doi: 10.1016/j.jcin.2019.08.041. Epub 2019 Dec 25. JACC Cardiovasc Interv. 2020. PMID: 31883714
-
Three Generations of Self-Expanding Transcatheter Aortic Valves: A Report From the STS/ACC TVT Registry.JACC Cardiovasc Interv. 2020 Jan 27;13(2):170-179. doi: 10.1016/j.jcin.2019.08.035. JACC Cardiovasc Interv. 2020. PMID: 31973793
-
Clinical Outcomes of the Self-Expandable Evolut R Valve Versus the Balloon-Expandable SAPIEN 3 Valve in Transcatheter Aortic Valve Implantation: A Meta-Analysis and Systematic Review.Cardiovasc Revasc Med. 2021 Apr;25:57-62. doi: 10.1016/j.carrev.2020.10.002. Epub 2020 Oct 16. Cardiovasc Revasc Med. 2021. PMID: 33160895
-
Transcathether aortic valve implantation with the new repositionable self-expandable Medtronic Evolut R vs. CoreValve system: evidence on the benefit of a meta-analytical approach.J Cardiovasc Med (Hagerstown). 2019 Apr;20(4):226-236. doi: 10.2459/JCM.0000000000000757. J Cardiovasc Med (Hagerstown). 2019. PMID: 30829877
Cited by
-
Impact of prosthesis oversizing on clinical outcomes of transcatheter aortic valve implantation using a self-expandable Evolut R valve.Egypt Heart J. 2024 Feb 12;76(1):20. doi: 10.1186/s43044-024-00450-0. Egypt Heart J. 2024. PMID: 38345661 Free PMC article.
-
Transcatheter Aortic Valve Replacement for Valvular Shock in an Extremely Large Annulus.JACC Case Rep. 2025 Mar 19;30(6 Pt 2):103545. doi: 10.1016/j.jaccas.2025.103545. JACC Case Rep. 2025. PMID: 40155146 Free PMC article.
-
Swimming in the OCEAN-TAVI of Large Annulus.JACC Asia. 2024 Sep 3;4(9):695-696. doi: 10.1016/j.jacasi.2024.08.001. eCollection 2024 Sep. JACC Asia. 2024. PMID: 39371620 Free PMC article.
-
Transcatheter Aortic Valve Implantation in Japanese Patients With Large Annulus: The OCEAN-TAVI Registry.JACC Asia. 2024 Aug 13;4(9):686-694. doi: 10.1016/j.jacasi.2024.07.002. eCollection 2024 Sep. JACC Asia. 2024. PMID: 39371621 Free PMC article.
References
-
- Popma JJ, Adams DH, Reardon MJ, Yakubov SJ, Kleiman NS, Heimansohn D, Hermiller J, Jr, Hughes GC, Harrison JK, Coselli J, et al. ; CoreValve United States Clinical Investigators. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol. 2014;63:1972–1981. doi: 10.1016/j.jacc.2014.02.556 - PubMed
-
- Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, Gleason TG, Buchbinder M, Hermiller J, Jr, Kleiman NS, et al. ; U.S. CoreValve Clinical Investigators. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370:1790–1798. doi: 10.1056/NEJMoa1400590 - PubMed
-
- Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Sondergaard L, Mumtaz M, Adams DH, Deeb GM, Maini B, Gada H, et al. ; SURTAVI Investigators. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376:1321–1331. doi: 10.1056/NEJMoa1700456 - PubMed
-
- Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O’Hair D, Bajwa T, Heiser JC, Merhi W, Kleiman NS, et al. ; Evolut Low Risk Trial Investigators. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380:1706–1715. doi: 10.1056/NEJMoa1816885 - PubMed
-
- Abdelghani M, Mankerious N, Allali A, Landt M, Kaur J, Sulimov DS, Merten C, Sachse S, Mehilli J, Neumann FJ, et al. . Bioprosthetic valve performance after transcatheter aortic valve replacement with self-expanding versus balloon-expandable valves in large versus small aortic valve annuli: insights from the CHOICE Trial and the CHOICE-extend registry. JACC Cardiovasc Interv. 2018;11:2507–2518. doi: 10.1016/j.jcin.2018.07.050 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

