Internalization of Angiotensin-(1-12) in Adult Retinal Pigment Epithelial-19 Cells
- PMID: 36944130
- PMCID: PMC10178934
- DOI: 10.1089/jop.2022.0139
Internalization of Angiotensin-(1-12) in Adult Retinal Pigment Epithelial-19 Cells
Abstract
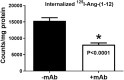
Purpose: Angiotensin-(1-12) [Ang-(1-12)] serves as a primary substrate to generate angiotensin II (Ang II) by angiotensin-converting enzyme and/or chymase suggests it may be an unrecognized source of Ang II-mediated microvascular complication in hypertension-mediated retinopathy. We investigated Ang-(1-12) expression and internalization in adult retinal pigment epithelial-19 (ARPE-19) cultured cells. We performed the internalization of Ang-(1-12) in ARPE-19 cells in the presence of a highly specific monoclonal antibody (mAb) developed against the C-terminal end of the Ang-(1-12) sequence. Methods: All experiments were performed in confluent ARPE-19 cells (passage 28-35). We employed high-performance liquid chromatography to purify radiolabeled, 125I-Ang-(1-12) and immuno-neutralization with Ang-(1-12) mAb to demonstrate Ang-(1-12)'s internalization in ARPE-19 cells. Internalization was also demonstrated by immunofluorescence (IF) method. Results: These procedures revealed internalization of an intact 125I-Ang-(1-12) in ARPE-19 cells. A significant reduction (∼53%, P < 0.0001) in 125I-Ang-(1-12) internalization was detected in APRE-19 cells in the presence of the mAb. IF staining experiments further confirms internalization of Ang-(1-12) into the cells from the extracellular culture medium. No endogenous expression was detected in the ARPE-19 cells. An increased intensity of IF staining was detected in cells exposed to 1.0 μM Ang-(1-12) compared with 0.1 μM. Furthermore, we found hydrolysis of Ang-(1-12) into Ang II by ARPE-19 cells' plasma membranes. Conclusions: Intact Ang-(1-12) peptide is internalized from the extracellular spaces in ARPE-19 cells and metabolized into Ang II. The finding that a selective mAb blocks cellular internalization of Ang-(1-12) suggests alternate therapeutic approaches to prevent/reduce the RPE cells Ang II burden.
Keywords: angiotensin II; angiotensin-(1–12); internalization; monoclonal antibody therapy; peptide metabolism; retinal pigment epithelial cells.
Conflict of interest statement
No competing financial interests exist.
Figures




References
-
- Nagata S, Kato J, Sasaki, K, et al. . Isolation and identification of proangiotensin-12, a possible component of the renin-angiotensin system. Biochem Biophys Res Commun 2006;350(4):1026–1031. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

