NPM1-Mutated Acute Myeloid Leukemia: Recent Developments and Open Questions
- PMID: 36944324
- PMCID: PMC10857804
- DOI: 10.1159/000530253
NPM1-Mutated Acute Myeloid Leukemia: Recent Developments and Open Questions
Abstract
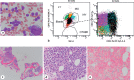
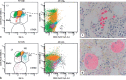
Somatic mutations in the nucleophosmin (NPM1) gene occur in approximately 30% of de novo acute myeloid leukemias (AMLs) and are relatively enriched in normal karyotype AMLs. Earlier World Health Organization (WHO) classification schema recognized NPM1-mutated AMLs as a unique subtype of AML, while the latest WHO and International Consensus Classification (ICC) now consider NPM1 mutations as AML-defining, albeit at different blast count thresholds. NPM1 mutational load correlates closely with disease status, particularly in the post-therapy setting, and therefore high sensitivity-based methods for detection of the mutant allele have proven useful for minimal/measurable residual disease (MRD) monitoring. MRD status has been conventionally measured by either multiparameter flow cytometry (MFC) and/or molecular diagnostic techniques, although recent data suggest that MFC data may be potentially more challenging to interpret in this AML subtype. Of note, MRD status does not predict patient outcome in all cases, and therefore a deeper understanding of the biological significance of MRD may be required. Recent studies have confirmed that NPM1-mutated cells rely on overexpression of HOX/MEIS1, which is dependent on the presence of the aberrant cytoplasmic localization of mutant NPM1 protein (NPM1c); this biology may explain the promising response to novel agents, including menin inhibitors and second-generation XPO1 inhibitors. In this review, these and other recent developments around NPM1-mutated AML, in addition to open questions warranting further investigation, will be discussed.
Keywords: Acute myeloid leukemia; Minimal residual disease; NPM1; Nucleophosmin.
© 2023 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The author acts as a subject matter expert/consultant for various clients via Guidepoint, LLC. and GLG, LLC. intermediaries.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

