Electrochemical Synthesis of Dimeric λ3-Bromane: Platform for Hypervalent Bromine(III) Compounds
- PMID: 36944352
- PMCID: PMC10071479
- DOI: 10.1021/acs.orglett.3c00405
Electrochemical Synthesis of Dimeric λ3-Bromane: Platform for Hypervalent Bromine(III) Compounds
Abstract
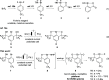
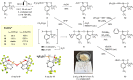
A straightforward and scalable approach to a previously unreported class of cyclic hypervalent Br(III) species capitalizes on the anodic oxidation of aryl bromide to dimeric benzbromoxole that serves as a versatile platform to access a range of structurally diverse Br(III) congeners such as acetoxy-, alkoxy-, and ethynyl-λ3-bromanes as well as diaryl-λ3-bromanes. The synthetic utility of dimeric λ3-bromane is exemplified by photoinduced Minisci-type heteroarylation reactions and benzylic oxidation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Winterson B.; Patra T.; Wirth T. Hypervalent Bromine(III) Compounds: Synthesis, Applications, Prospects. Synthesis 2022, 54, 1261–1271. 10.1055/a-1675-8404. - DOI
- Miyamoto K.Chemistry of Hypervalent Bromine. In PATAI’S Chemistry of Functional Groups; Rappoport Z., Ed.; John Wiley & Sons, Ltd: Chichester, U.K., 2018; pp 1–25.
- Farooq U.; Shah A.-H. A.; Wirth T. Hypervalent Bromine Compounds: Smaller, More Reactive Analogues of Hypervalent Iodine Compounds. Angew. Chem., Int. Ed. 2009, 48, 1018–1020. 10.1002/anie.200805027. - DOI - PubMed
-
- Ochiai M.; Yoshimura A.; Miyamoto K.; Hayashi S.; Nakanishi W. Hypervalent λ3-Bromane Strategy for Baeyer–Villiger Oxidation: Selective Transformation of Primary Aliphatic and Aromatic Aldehydes to Formates, Which Is Missing in the Classical Baeyer–Villiger Oxidation. J. Am. Chem. Soc. 2010, 132, 9236–9239. 10.1021/ja104330g. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

