Virtual and Reality: An Analysis of the UCLA Virtual Crossmatch Exchanges
- PMID: 36944607
- PMCID: PMC10358445
- DOI: 10.1097/TP.0000000000004586
Virtual and Reality: An Analysis of the UCLA Virtual Crossmatch Exchanges
Abstract
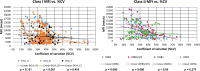
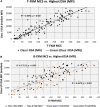
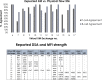
The "virtual" crossmatch (VXM) has become a critical tool to predict the compatibility between an organ donor and a potential recipient. Yet, nonstandardized laboratory practice can lead to variability in VXM interpretation. Therefore, UCLA's VXM Exchange survey was designed to understand factors that influence the variability of VXM prediction in the presence of HLA donor-specific antibody (DSA). Thirty-six donor blood samples and 72 HLA reference sera were sent to 35 participating laboratories to perform HLA antibody testing, flow crossmatch (FXM), and VXM from 2014 to 2019, consisting of 144 T/B-cell FXM pairs and 112 T/B-cell VXM pairs. In the FXM survey, 86% T-cell FXM and 84% B-cell FXM achieved >80% concordance among laboratories. In the VXM survey, 81% T-cell VXM and 80% VXM achieved >80% concordance. The concordance between FXM and VXM was 79% for T cell and 87% for B cell. The consensus between VXM and FXM was high with strong DSA. However, significant variability was observed in sera with (1) very high titer antibodies that exit prozone effect; (2) weak-to-moderate DSA, particularly in the presence of multiple weak DSAs; and (3) DSA against lowly expressed antigens. With the increasing use the VXM, standardization and continuous learning via exchange surveys will provide better understanding and quality controls for VXM to improve accuracy across all centers.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Comment in
-
The Virtual Crossmatch: What's in a Name?Transplantation. 2023 Oct 1;107(10):e273. doi: 10.1097/TP.0000000000004723. Epub 2023 Sep 25. Transplantation. 2023. PMID: 37749816 No abstract available.
References
-
- Wiebe C, Gibson IW, Blydt-Hansen TD, et al. . Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant. 2012;12:1157–1167. - PubMed
-
- Smith JD, Banner NR, Hamour IM, et al. . De novo donor HLA-specific antibodies after heart transplantation are an independent predictor of poor patient survival. Am J Transplant. 2011;11:312–319. - PubMed

