The interaction between ferroptosis and inflammatory signaling pathways
- PMID: 36944609
- PMCID: PMC10030804
- DOI: 10.1038/s41419-023-05716-0
The interaction between ferroptosis and inflammatory signaling pathways
Abstract
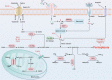
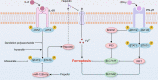
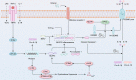
Ferroptosis is an iron-dependent regulated cell death driven by excessive lipid peroxidation. Inflammation is one common and effective physiological event that protects against various stimuli to maintain tissue homeostasis. However, the dysregulation of inflammatory responses can cause imbalance of the immune system, cell dysfunction and death. Recent studies have pointed out that activation of inflammation, including the activation of multiple inflammation-related signaling pathways, can lead to ferroptosis. Among the related signal transduction pathways, we focused on five classical inflammatory pathways, namely, the JAK-STAT, NF-κB, inflammasome, cGAS-STING and MAPK signaling pathways, and expounded on their roles in ferroptosis. To date, many agents have shown therapeutic effects on ferroptosis-related diseases by modulating the aforementioned pathways in vivo and in vitro. Moreover, the regulatory effects of these pathways on iron metabolism and lipid peroxidation have been described in detail, contributing to further understanding of the pathophysiological process of ferroptosis. Taken together, targeting these pathways related to inflammation will provide appropriate ways to intervene ferroptosis and diseases.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

