DT-0111: a novel P2X3 receptor antagonist
- PMID: 36944825
- PMCID: PMC10539268
- DOI: 10.1007/s11302-023-09930-5
DT-0111: a novel P2X3 receptor antagonist
Abstract
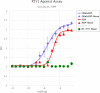
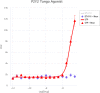
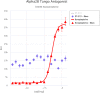
Extracellular adenosine 5'-triphosphate (ATP) acts as an autocrine and paracrine agent, the actions of which on affected cells are mediated by P2 receptors (P2R), which include trans cell-membrane cationic channels (P2XRs), and G protein coupled receptors (P2YRs). The mammalian P2X receptors form homotrimeric or heterotrimeric cationic channels, each of which contains three ATP-binding sites. There are seven homotrimeric P2X receptors (P2X1-7) and three heteromeric (P2X2/P2X3, P2X4/P2X6, P2X1/P2X5). In the lungs and airways, ATP activates P2X3 and P2X2/3 receptors (P2X3R, P2X2/3R, respectively) localized on vagal sensory nerve terminals resulting in bronchoconstriction, and cough, and probably also localized release of pro-inflammatory neuropeptides via the axon reflex. Currently, several P2X3R and P2X2/3R antagonists are being developed as drug-candidates for the treatment of chronic cough. This report presents the receptor affinity data of a novel water-soluble small molecule, DT-0111, that acts as a selective P2X3R antagonist.
Keywords: Adenosine 5’-triphosphate; Asthma; Bronchodilation; COPD; Cough; Vagus nerve.
© 2023. The Author(s), under exclusive licence to Springer Nature B.V.
Conflict of interest statement
Drs. Pelleg and Mahadevan are the CEOs of Danmir Therapeutics, LLC and Organix, Inc. respectively, which together are developing DT-0111 as a drug candidate for the treatment of several pulmonary disorders. Drs. Sirtori and Rolland declare no conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

