Stellate ganglion block ameliorated central post-stroke pain with comorbid anxiety and depression through inhibiting HIF-1α/NLRP3 signaling following thalamic hemorrhagic stroke
- PMID: 36944982
- PMCID: PMC10031944
- DOI: 10.1186/s12974-023-02765-2
Stellate ganglion block ameliorated central post-stroke pain with comorbid anxiety and depression through inhibiting HIF-1α/NLRP3 signaling following thalamic hemorrhagic stroke
Abstract
Background: Central post-stroke pain (CPSP) is an intractable and disabling central neuropathic pain that severely affects patients' lives, well-being, and socialization abilities. However, CPSP has been poorly studied mechanistically and its treatment remains challenging. Here, we used a rat model of CPSP induced by thalamic hemorrhage to investigate its underlying mechanisms and the effect of stellate ganglion block (SGB) on CPSP and emotional comorbidities.
Methods: Thalamic hemorrhage was produced by injecting collagenase IV into the ventral-posterolateral nucleus (VPL) of the right thalamus. The up-and-down method with von Frey hairs was used to measure the mechanical allodynia. Behavioral tests were carried out to examine depressive and anxiety-like behaviors including the open field test (OFT), elevated plus maze test (EPMT), novelty-suppressed feeding test (NSFT), and forced swim test (FST). The peri-thalamic lesion tissues were collected for immunofluorescence, western blotting, and enzyme-linked immunosorbent assay (ELISA). Genetic knockdown of thalamic hypoxia-inducible factor-1α (HIF-1α) and NOD-like receptor thermal protein domain associated protein 3 (NLRP3) with microinjection of HIF-1α siRNA and NLRP3 siRNA into the VPL of thalamus were performed 3 days before collagenase injection into the same regions. Microinjection of lificiguat (YC-1) and MCC950 into the VPL of thalamus were administrated 30 min before the collagenase injection in order to inhibited HIF-1α and NLRP3 pharmacologically. Repetitive right SGB was performed daily for 5 days and laser speckle contrast imaging (LSCI) was conducted to examine cerebral blood flow.
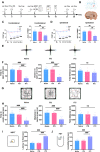
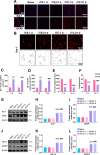
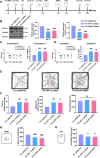
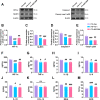
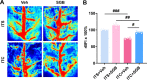
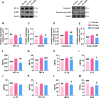
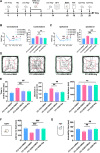
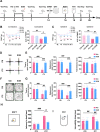
Results: Thalamic hemorrhage caused persistent mechanical allodynia and anxiety- and depression-like behaviors. Accompanying the persistent mechanical allodynia, the expression of HIF-1α and NLRP3, as well as the activities of microglia and astrocytes in the peri-thalamic lesion sites, were significantly increased. Genetic knockdown of thalamic HIF-1α and NLRP3 significantly attenuated mechanical allodynia and anxiety- and depression-like behaviors following thalamic hemorrhage. Further studies revealed that intra-thalamic injection of YC-1, or MCC950 significantly suppressed the activation of microglia and astrocytes, the release of pro-inflammatory cytokines, the upregulation of malondialdehyde (MDA), and the downregulation of superoxide dismutase (SOD), as well as mechanical allodynia and anxiety- and depression-like behaviors following thalamic hemorrhage. In addition, repetitive ipsilateral SGB significantly restored the upregulated HIF-1α/NLRP3 signaling and the hyperactivated microglia and astrocytes following thalamic hemorrhage. The enhanced expression of pro-inflammatory cytokines and the oxidative stress in the peri-thalamic lesion sites were also reversed by SGB. Moreover, LSCI showed that repetitive SGB significantly increased cerebral blood flow following thalamic hemorrhage. Most strikingly, SGB not only prevented, but also reversed the development of mechanical allodynia and anxiety- and depression-like behaviors induced by thalamic hemorrhage. However, pharmacological activation of thalamic HIF-1α and NLRP3 with specific agonists significantly eliminated the therapeutic effects of SGB on mechanical allodynia and anxiety- and depression-like behaviors following thalamic hemorrhage.
Conclusion: This study demonstrated for the first time that SGB could improve CPSP with comorbid anxiety and depression by increasing cerebral blood flow and inhibiting HIF-1α/NLRP3 inflammatory signaling.
Keywords: Anxiety; Central post-stroke pain; Depression; Hypoxia-inducible factor 1α; NLRP3; Neuroinflammation; Stellate ganglion block; Thalamus.
© 2023. The Author(s).
Conflict of interest statement
The authors have no conflict or competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
- 2022J01490/Natural Science Foundation of Fujian Province of China
- 2021J011264/Natural Science Foundation of Fujian Province of China
- 81801101/National Natural Science Foundation of China
- 2020Y9043/the Joint Funds for the Innovation of Science and Technology of Fujian Province
- 20QNPY073/the Army's Youth Cultivation Program
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

