Placental mesenchymal stem cells ameliorate NLRP3 inflammasome-induced ovarian insufficiency by modulating macrophage M2 polarization
- PMID: 36945010
- PMCID: PMC10029285
- DOI: 10.1186/s13048-023-01136-y
Placental mesenchymal stem cells ameliorate NLRP3 inflammasome-induced ovarian insufficiency by modulating macrophage M2 polarization
Abstract
Background: Premature ovarian insufficiency (POI) is a common clinical problem, however, there are currently no effective therapies. Pyroptosis induced by the NLRP3 inflammasome is considered a possible mechanism of POI. Placental mesenchymal stem cells (PMSCs) have excellent immunomodulatory potential and offer a promising method for treating POI.
Methods: Female Sprague-Dawley rats were randomly divided into four treatment groups: control (no POI), POI with no PMSCs, POI with PMSCs transplant, and POI with hormones (estrogen + progesterone) as positive control. POI was induced by exposure to 4-vinylcyclohexene diepoxide (VCD) for 15 days. After four weeks, all animals were euthanized and examined for pathology. Hormone levels were measured and ovarian function was evaluated in relation to the estrous cycle. Levels of NLRP3 inflammasome pathway proteins were determined by immunohistochemistry and western blot.
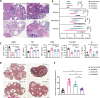
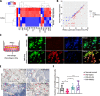
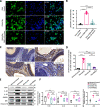
Results: VCD significantly damaged rat follicles at different estrous stages. Injection of human PMSCs improved ovarian function and reproductive ability of POI rats compared to the sham and hormone groups. Our data also showed that PMSCs markedly suppress cell pyroptosis via downregulation of the NLRP3 inflammasome, caspase-1, IL-1β and IL-18 compared to the other two groups. The human PMSCs increased the expression of IL-4 and IL-10 and decreased pro-inflammatory factors by phenotypic changes in macrophages.
Conclusions: Our findings revealed a novel mechanism of follicular dysfunction and ovarian fibrosis via activation of the NLRP3 inflammasome followed by secretion of pro-inflammatory factors. Transplantation of PMSCs into POI rats suppressed pro-inflammatory factor production, NLRP3 inflammasome formation and pyroptosis, and improved ovarian function.
Keywords: Inflammasome; Interferon-γ; Macrophage; NLRP3; Placental mesenchymal stem cells; Premature ovarian insufficiency; Pyroptosis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Shin EY, Kim DS, Lee MJ, Lee AR, Shim SH, Baek SW, Han DK, Lee DR. Prevention of chemotherapy-induced premature ovarian insufficiency in mice by scaffold-based local delivery of human embryonic stem cell-derived mesenchymal progenitor cells. Stem Cell Res Ther. 2021;12(1):431. doi: 10.1186/s13287-021-02479-3. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

