ALDOC- and ENO2- driven glucose metabolism sustains 3D tumor spheroids growth regardless of nutrient environmental conditions: a multi-omics analysis
- PMID: 36945054
- PMCID: PMC10031988
- DOI: 10.1186/s13046-023-02641-0
ALDOC- and ENO2- driven glucose metabolism sustains 3D tumor spheroids growth regardless of nutrient environmental conditions: a multi-omics analysis
Abstract
Background: Metastases are the major cause of cancer-related morbidity and mortality. By the time cancer cells detach from their primary site to eventually spread to distant sites, they need to acquire the ability to survive in non-adherent conditions and to proliferate within a new microenvironment in spite of stressing conditions that may severely constrain the metastatic process. In this study, we gained insight into the molecular mechanisms allowing cancer cells to survive and proliferate in an anchorage-independent manner, regardless of both tumor-intrinsic variables and nutrient culture conditions.
Methods: 3D spheroids derived from lung adenocarcinoma (LUAD) and breast cancer cells were cultured in either nutrient-rich or -restricted culture conditions. A multi-omics approach, including transcriptomics, proteomics, and metabolomics, was used to explore the molecular changes underlying the transition from 2 to 3D cultures. Small interfering RNA-mediated loss of function assays were used to validate the role of the identified differentially expressed genes and proteins in H460 and HCC827 LUAD as well as in MCF7 and T47D breast cancer cell lines.
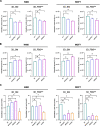
Results: We found that the transition from 2 to 3D cultures of H460 and MCF7 cells is associated with significant changes in the expression of genes and proteins involved in metabolic reprogramming. In particular, we observed that 3D tumor spheroid growth implies the overexpression of ALDOC and ENO2 glycolytic enzymes concomitant with the enhanced consumption of glucose and fructose and the enhanced production of lactate. Transfection with siRNA against both ALDOC and ENO2 determined a significant reduction in lactate production, viability and size of 3D tumor spheroids produced by H460, HCC827, MCF7, and T47D cell lines.
Conclusions: Our results show that anchorage-independent survival and growth of cancer cells are supported by changes in genes and proteins that drive glucose metabolism towards an enhanced lactate production. Notably, this finding is valid for all lung and breast cancer cell lines we have analyzed in different nutrient environmental conditions. broader Validation of this mechanism in other cancer cells of different origin will be necessary to broaden the role of ALDOC and ENO2 to other tumor types. Future in vivo studies will be necessary to assess the role of ALDOC and ENO2 in cancer metastasis.
Keywords: ALDOC; Breast cancer; ENO2; Glucose metabolism; Lung cancer; Metastasis; Omics; Tumor spheroids.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







References
-
- Raimo D, di Raimo T, de Santis E, Coppola L, Rosario D’andrea M, Angelini F. Circulating tumor cells and the metastatic process: the complexity of malignancy. J Cancer Metastasis Treat. 2018;4:54. doi: 10.20517/2394-4722.2018.50. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

