Impact of IgG subclass on monoclonal antibody developability
- PMID: 36945111
- PMCID: PMC10038059
- DOI: 10.1080/19420862.2023.2191302
Impact of IgG subclass on monoclonal antibody developability
Abstract
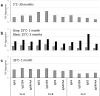
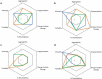
IgG-based monoclonal antibody therapeutics, which are mainly IgG1, IgG2, and IgG4 subclasses or related variants, have dominated the biotherapeutics field for decades. Multiple laboratories have reported that the IgG subclasses possess different molecular characteristics that can affect their developability. For example, IgG1, the most popular IgG subclass for therapeutics, is known to have a characteristic degradation pathway related to its hinge fragility. However, there remains a paucity of studies that systematically evaluate the IgG subclasses on manufacturability and long-term stability. We thus conducted a systematic study of 12 mAbs derived from three sets of unrelated variable regions, each cloned into IgG1, an IgG1 variant with diminished effector functions, IgG2, and a stabilized IgG4 variant with further reduced FcγR interaction, to evaluate the impact of IgG subclass on manufacturability and high concentration stability in a common formulation buffer matrix. Our evaluation included Chinese hamster ovary cell productivity, host cell protein removal efficiency, N-linked glycan structure at the conserved N297 Fc position, solution appearance at high concentration, and aggregate growth, fragmentation, charge variant profile change, and post-translational modification upon thermal stress conditions or long-term storage at refrigerated temperature. Our results elucidated molecular attributes that are common to all IgG subclasses, as well as those that are unique to certain Fc domains, providing new insight into the effects of IgG subclass on antibody manufacturability and stability. These learnings can be used to enable a balanced decision on IgG subclass selection for therapeutic antibodies and aid in acceleration of their product development process.
Keywords: Aggregates; IgG subclass; Monoclonal antibodies; charge variants; developability; fragments; host cell protein; post-translational modification; productivity; stability.
Conflict of interest statement
All authors in this report except Yu Tang are current employees of Eli Lilly and Company. All the data in this report were generated at Eli Lilly and Company, Indianapolis, IN. The authors do not have any conflict of interest or financial disclosure to report.
Figures




References
-
- Skamris T, Tian X, Thorolfsson M, Karkov HS, Rasmussen HB, Langkilde AE, Vestergaard B. Monoclonal antibodies follow distinct aggregation pathways during production-relevant acidic incubation and neutralization. Pharm Res. 2016;33:716–28. doi: 10.1007/s11095-015-1821-0. PMID: 26563206. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
