A Histological and Clinical Study of MatriDerm® Use in Burn Reconstruction
- PMID: 36945134
- PMCID: PMC10483478
- DOI: 10.1093/jbcr/irad024
A Histological and Clinical Study of MatriDerm® Use in Burn Reconstruction
Abstract
Dermal substitutes are well established in the reconstructive ladder. MatriDerm® (Dr. Otto Suwelack Skin & Health Care AG, Billerbeck, Germany) is a single-layer dermal substitute composed of a bovine collagen (type I, III, and V) and elastin hydrolysate, that allows for immediate split-thickness skin grafting (SSG). The aim of this study was to histologically characterize the integration of MatriDerm® when used during burns surgery reconstruction. Eight subjects with nine burn scars and one acute burn wound underwent reconstruction with MatriDerm® and an immediate SSG. MatriDerm® integration and skin graft take were assessed with serial biopsies performed at weeks 1, 2, 3, and 4 and months 2, 3, 6, 9, and 12. Biopsies were assessed with standard special stains and immunohistochemistry, and representative slides were imaged with a transmission electron microscope. Patient satisfaction and clinical scar outcome were assessed with the Vancouver Scar Scale and a patient questionnaire. Histological analysis showed similar stages of wound healing as shown in other dermal templates but on a different timescale. There is early evidence of vascularization and an inflammatory infiltrate in the first 2 weeks. MatriDerm® is resorbed earlier than other dermal substitutes, with evidence of resorption at week 3, to be completely replaced by a neodermis at 2 months. The use of MatriDerm® in reconstruction with immediate skin grafting is supported histologically with early evidence of vascularization to support an epidermal autograft. Future histological studies may help further characterize the ideal dermal substitute.
© The Author(s) 2023. Published by Oxford University Press on behalf of the American Burn Association.
Figures
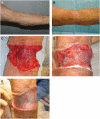
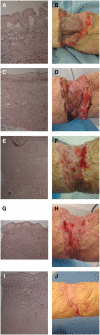
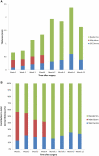
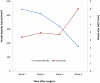

References
-
- Stern R, McPherson M, Longaker MT.. Histologic study of artificial skin used in the treatment of full-thickness thermal injury. J Burn Care Rehabil 1990;11:7–13. - PubMed
-
- Moiemen NS, Staiano JJ, Ojeh NO, Thway Y, Frame JD.. Reconstructive surgery with a dermal regeneration template: clinical and histologic study. Plast Reconstr Surg 2001;108:93–103. - PubMed
-
- Dantzer E, Braye FM.. Reconstructive surgery using an artificial dermis (Integra): results with 39 grafts. Br J Plast Surg 2001;54:659–64. - PubMed
-
- Petersen W, Rahmanian-Schwarz A, Werner JOet al. The use of collagen-based matrices in the treatment of full-thickness wounds. Burns 2016;42:1257–64. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

