This is a preprint.
Antibody-directed extracellular proximity biotinylation reveals Contactin-1 regulates axo-axonic innervation of axon initial segments
- PMID: 36945454
- PMCID: PMC10028829
- DOI: 10.1101/2023.03.06.531378
Antibody-directed extracellular proximity biotinylation reveals Contactin-1 regulates axo-axonic innervation of axon initial segments
Update in
-
Antibody-directed extracellular proximity biotinylation reveals that Contactin-1 regulates axo-axonic innervation of axon initial segments.Nat Commun. 2023 Oct 26;14(1):6797. doi: 10.1038/s41467-023-42273-8. Nat Commun. 2023. PMID: 37884508 Free PMC article.
Abstract
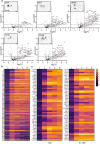
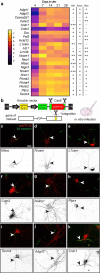
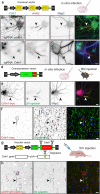
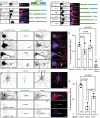
Axon initial segment (AIS) cell surface proteins mediate key biological processes in neurons including action potential initiation and axo-axonic synapse formation. However, few AIS cell surface proteins have been identified. Here, we used antibody-directed proximity biotinylation to define the cell surface proteins in close proximity to the AIS cell adhesion molecule Neurofascin. To determine the distributions of the identified proteins, we used CRISPR-mediated genome editing for insertion of epitope tags in the endogenous proteins. We found Contactin-1 (Cntn1) among the previously unknown AIS proteins we identified. Cntn1 is enriched at the AIS through interactions with Neurofascin and NrCAM. We further show that Cntn1 contributes to assembly of the AIS-extracellular matrix, and is required for AIS axo-axonic innervation by inhibitory basket cells in the cerebellum and inhibitory chandelier cells in the cortex.
Conflict of interest statement
COMPETING INTERESTS The authors declare no competing interests.
Figures




References
-
- Rasband M. N. The axon initial segment and the maintenance of neuronal polarity. Nat Rev Neurosci 11, 552–562 (2010). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
