This is a preprint.
POTENTIATION OF CORTICO-SPINAL OUTPUT VIA TARGETED ELECTRICAL STIMULATION OF THE MOTOR THALAMUS
- PMID: 36945514
- PMCID: PMC10029067
- DOI: 10.1101/2023.03.08.23286720
POTENTIATION OF CORTICO-SPINAL OUTPUT VIA TARGETED ELECTRICAL STIMULATION OF THE MOTOR THALAMUS
Update in
-
Potentiation of cortico-spinal output via targeted electrical stimulation of the motor thalamus.Nat Commun. 2024 Oct 1;15(1):8461. doi: 10.1038/s41467-024-52477-1. Nat Commun. 2024. PMID: 39353911 Free PMC article.
Abstract
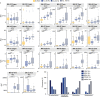
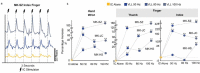
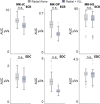
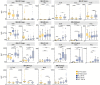
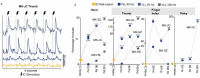
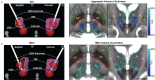
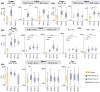
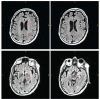
Cerebral white matter lesions prevent cortico-spinal descending inputs from effectively activating spinal motoneurons, leading to loss of motor control. However, in most cases, the damage to cortico-spinal axons is incomplete offering a potential target for new therapies aimed at improving volitional muscle activation. Here we hypothesized that, by engaging direct excitatory connections to cortico-spinal motoneurons, stimulation of the motor thalamus could facilitate activation of surviving cortico-spinal fibers thereby potentiating motor output. To test this hypothesis, we identified optimal thalamic targets and stimulation parameters that enhanced upper-limb motor evoked potentials and grip forces in anesthetized monkeys. This potentiation persisted after white matter lesions. We replicated these results in humans during intra-operative testing. We then designed a stimulation protocol that immediately improved voluntary grip force control in a patient with a chronic white matter lesion. Our results show that electrical stimulation targeting surviving neural pathways can improve motor control after white matter lesions.
Conflict of interest statement
Competing interests The authors declare no conflicts of interests in relation to this work.
Figures










References
-
- Lemon R. N. Descending pathways in motor control. Annu. Rev. Neurosci. 31, 195–218 (2008). - PubMed
-
- Zaloshnja E., Miller T., Langlois J. A. & Selassie A. W. Prevalence of long-term disability from traumatic brain injury in the civilian population of the United States, 2005. The Journal of head trauma rehabilitation 23, 394–400 (2008). - PubMed
-
- Simonetta-Moreau M. Non-invasive brain stimulation (NIBS) and motor recovery after stroke. Annals of physical and rehabilitation medicine 57, 530–542 (2014). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
