This is a preprint.
A pilot study on metabolomic characterization of human glioblastomas and patient plasma
- PMID: 36945517
- PMCID: PMC10029122
- DOI: 10.21203/rs.3.rs-2662020/v1
A pilot study on metabolomic characterization of human glioblastomas and patient plasma
Update in
-
Metabolomic characterization of human glioblastomas and patient plasma: a pilot study.F1000Res. 2024 Sep 19;13:98. doi: 10.12688/f1000research.143642.5. eCollection 2024. F1000Res. 2024. PMID: 39371551 Free PMC article.
Abstract
Purpose: To determine whether recurrent GBMs are metabolically distinct from primary GBM, and whether patient plasma can be used as a liquid biopsy to reflect this difference.
Methods: In a single center cohort study, tissue and blood samples from 15 patients with glioblastoma (9 glioblastoma tissues at diagnosis, 3 pairs of tissue, and 6 pairs of plasma specimens at diagnosis and at recurrence) were analyzed.
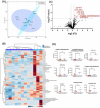
Results: Several metabolites had significant alternations in both tumor and plasma specimens. In the tissue, the following representative metabolites had a significant increase in peak intensity at recurrence compared to diagnosis: N-alpha-methylhistamine (p = 0.037), glycerol-3-phosphate (p = 0.029), phosphocholine (p = 0.045), and succinic acid (p = 0.025). In patient plasma, metabolites that significantly increased at recurrence included: 2,4-difluorotoluene (p = 0.031), diatrizoic acid (p = 0.032), indole-3-acetate with (p = 0.029), urea (P = 0.025), pseudouridine (p = 0.042), and maltose (p = 0.035). Metabolites that significantly decreased in plasma at recurrence were: eicosenoic acid (p = 0.017), glucose-1-phosphate (p = 0.017), FA 18:2 (linoleic acid) (p = 0.017), arginine (p = 0.036), fatty acids 20:3 (homo-gamma-linolenic acid (p = 0.036), galactosamine (p = 0.007), and FA 18:3 (linolenic acid) (P = 0.012). Principal component analysis showed that the metabolomic profiles differ between tumor tissue and patient plasma.
Conclusions: Our data suggest that metabolomic profiles of human GBM tissue and patient plasma differ at diagnosis and at recurrence. Many metabolites involved in tumorigenesis and metabolomic flexibility were identified. A larger study using targeted metabolomic assay is warranted to measure the levels of these metabolites, which will help identify the metabolomic signatures in both GBM tissue and patient plasma for risk stratification, clinical outcome prediction, and development of new adjuvant metabolomic-targeting therapy.
Keywords: biomarker; feasibility; glioblastoma; pilot; untargeted metabolomics.
Figures



References
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for R, Treatment of Cancer Brain T, Radiotherapy G, National Cancer Institute of Canada Clinical Trials G (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352: 987–996 doi:10.1056/NEJMoa043330 - DOI - PubMed
-
- Hanahan D (2022) Hallmarks of Cancer: New Dimensions. Cancer Discov 12:31–46. 10.1158/2159-8290.CD-21-1059 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

