This is a preprint.
Targeted therapies prime oncogene-driven lung cancers for macrophage-mediated destruction
- PMID: 36945559
- PMCID: PMC10028834
- DOI: 10.1101/2023.03.03.531059
Targeted therapies prime oncogene-driven lung cancers for macrophage-mediated destruction
Update in
-
Targeted therapies prime oncogene-driven lung cancers for macrophage-mediated destruction.J Clin Invest. 2024 Mar 14;134(9):e169315. doi: 10.1172/JCI169315. J Clin Invest. 2024. PMID: 38483480 Free PMC article.
Abstract
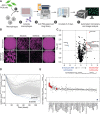
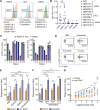
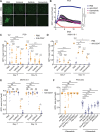
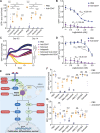
Macrophage immune checkpoint inhibitors, such as anti-CD47 antibodies, show promise in clinical trials for solid and hematologic malignancies. However, the best strategies to use these therapies remain unknown and ongoing studies suggest they may be most effective when used in combination with other anticancer agents. Here, we developed a novel screening platform to identify drugs that render lung cancer cells more vulnerable to macrophage attack, and we identified therapeutic synergy exists between genotype-directed therapies and anti-CD47 antibodies. In validation studies, we found the combination of genotype-directed therapies and CD47 blockade elicited robust phagocytosis and eliminated persister cells in vitro and maximized anti-tumor responses in vivo. Importantly, these findings broadly applied to lung cancers with various RTK/MAPK pathway alterations-including EGFR mutations, ALK fusions, or KRASG12C mutations. We observed downregulation of β2-microglobulin and CD73 as molecular mechanisms contributing to enhanced sensitivity to macrophage attack. Our findings demonstrate that dual inhibition of the RTK/MAPK pathway and the CD47/SIRPa axis is a promising immunotherapeutic strategy. Our study provides strong rationale for testing this therapeutic combination in patients with lung cancers bearing driver mutations.
Conflict of interest statement
Conflict-of-interest statement: K.V. is currently an employee and equity owner of DEM Biopharma. D.Y. is a co-founder, SAB member, and equity holder of DEM Biopharma. N.P. is a current employee of Bristol-Myers Squibb. A.N.H. has received research support from Amgen, Blueprint Medicines, BridgeBio, Bristol-Myers Squibb, C4 Therapeutics, Eli Lilly, Nuvalent, Pfizer, Roche/Genentech, Scorpion Therapeutics; has served as a compensated consultant for Nuvalent, Tolremo Therapeutics, Engine Biosciences and TigaTx. K.W. declares relevant relationships pertaining to macrophage-directed therapies including patents and royalties (Stanford University, Whitehead Institute, Gilead Sciences); co-founder, SAB member, and equity holder (ALX Oncology, DEM Biopharma); scientific advisor (Carisma Therapeutics). K.W. reports stock ownership of Ginkgo Bioworks. K.W., A.H., K.D.V, J.L.A, D.Y., and A.M. have filed U.S. patent applications related to this work. The other authors have declared that no conflict of interest exists.
Figures

References
-
- Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378(2):113–25. - PubMed
-
- Tan AC, and Tan DSW. Targeted Therapies for Lung Cancer Patients With Oncogenic Driver Molecular Alterations. J Clin Oncol. 2022;40(6):611–25. - PubMed
-
- Shaw AT, Bauer TM, de Marinis F, Felip E, Goto Y, Liu G, et al. First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer. N Engl J Med. 2020;383(21):2018–29. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
