This is a preprint.
A molecular glue approach to control the half-life of CRISPR-based technologies
- PMID: 36945568
- PMCID: PMC10028966
- DOI: 10.1101/2023.03.12.531757
A molecular glue approach to control the half-life of CRISPR-based technologies
Update in
-
A Molecular Glue Approach to Control the Half-Life of CRISPR-Based Technologies.J Am Chem Soc. 2025 Jul 9;147(27):23844-23856. doi: 10.1021/jacs.5c06230. Epub 2025 Jun 30. J Am Chem Soc. 2025. PMID: 40586242 Free PMC article.
Abstract
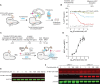
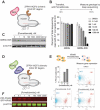
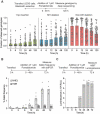
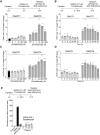
Cas9 is a programmable nuclease that has furnished transformative technologies, including base editors and transcription modulators (e.g., CRISPRi/a), but several applications of these technologies, including therapeutics, mandatorily require precision control of their half-life. For example, such control can help avert any potential immunological and adverse events in clinical trials. Current genome editing technologies to control the half-life of Cas9 are slow, have lower activity, involve fusion of large response elements (> 230 amino acids), utilize expensive controllers with poor pharmacological attributes, and cannot be implemented in vivo on several CRISPR-based technologies. We report a general platform for half-life control using the molecular glue, pomalidomide, that binds to a ubiquitin ligase complex and a response-element bearing CRISPR-based technology, thereby causing the latter's rapid ubiquitination and degradation. Using pomalidomide, we were able to control the half-life of large CRISPR-based technologies (e.g., base editors, CRISPRi) and small anti-CRISPRs that inhibit such technologies, allowing us to build the first examples of on-switch for base editors. The ability to switch on, fine-tune and switch-off CRISPR-based technologies with pomalidomide allowed complete control over their activity, specificity, and genome editing outcome. Importantly, the miniature size of the response element and favorable pharmacological attributes of the drug pomalidomide allowed control of activity of base editor in vivo using AAV as the delivery vehicle. These studies provide methods and reagents to precisely control the dosage and half-life of CRISPR-based technologies, propelling their therapeutic development.
Conflict of interest statement
The authors declare the following competing financial interest: Broad Institute has filed patents claiming inventions to genome editing methods in this manuscript. M.J. has received consulting fees from RA Ventures. M.K. holds equity in and serves on the scientific advisory boards of Engine Biosciences, Casma Therapeutics, Cajal Neuroscience, and Alector and advises Modulo Bio and Recursion Therapeutics. M.K. is an inventor on US patent 11,354,933 related to CRISPRi and CRISPRa screening. L.P. has financial interests in Edilytics, Excelsior Genomics and SeQure Dx. L.P.’s interests were reviewed and are managed by Massachusetts General Hospital and Partners HealthCare in accordance with their conflict-of-interest policies. D.R.L. is a consultant for Prime Medicine, Beam Therapeutics, Pairwise Plants, Chroma Medicine, and Nvelop Therapeutics, companies that use or deliver genome editing or epigenome engineering agents and owns equity in these companies. B.L.E. has received research funding from Celgene, Deerfield, Novartis, and Calico and consulting fees from GRAIL. B.L.E. is a member of the scientific advisory board and shareholder for Neomorph Inc., TenSixteen Bio, Skyhawk Therapeutics, and Exo Therapeutics. A.C. is the scientific founder and scientific advisory board member of Photys Therapeutics.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
