Comparison of survival outcomes according of patients with metastatic gastric cancer receiving trastuzumab with systemic chemotherapy
- PMID: 36945715
- PMCID: PMC9942733
- DOI: 10.14216/kjco.20011
Comparison of survival outcomes according of patients with metastatic gastric cancer receiving trastuzumab with systemic chemotherapy
Abstract
Purpose: Currently, trastuzumab plus chemotherapy is the standard first-line therapy for human epidermal growth factor receptor 2 (HER2)-positive advanced or metastatic gastric cancer (mGC) or esophagogastric junction cancer. However, it is not clear whether the prognosis of HER2-positive mGC treated with trastuzumab plus chemotherapy is better than that of HER2-negative mGC treated with chemotherapy as the first-line therapy.
Methods: We performed a retrospective study comparing the prognosis of mGC according to first-line treatment with trastuzumab plus chemotherapy or chemotherapy only, at the Korea Cancer Center Hospital from 2011 to 2018. The Kaplan-Meier method and Cox proportional hazards model were used for univariate and multivariate survival analyses.
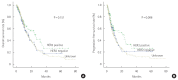
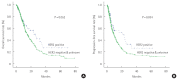
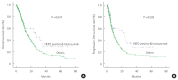
Results: The median overall survival of trastuzumab group was 26.1 months and that of chemotherapy group was 14.8 months (P=0.047). Trastuzumab group had a longer median progression-free survival than chemotherapy group (23.4 vs. 9.2 months, P=0.026). By univariate analysis, sex, age, World Health Organization (WHO) histology, HER2 status, primary tumor site, extent of disease, number of lesions, number of metastatic, measurability of disease, prior gastrectomy, and chemotherapy group are statistically significant. Using multivariate analysis, number of lesions, number of metastatic, prior gastrectomy, and trastuzumab group (hazard ratio, 0.594; 95% confidence interval, 0.384-0.921; P=0.020) were found to be independent prognostic factors of overall survival.
Conclusion: The result suggests prognosis of HER2-positive mGC treated by trastuzumab plus chemotherapy could be better than that of HER2-negative mGC treated by chemotherapy only. Well-designed prospective cohort studies are needed to confirm the results of this study. HER2 testing should be performed routinely in all patients newly diagnosed with mGC.
Keywords: Drug therapy; Esophagogastric junction; Stomach; Survival; Trastuzumab.
Copyright © 2020 Korean Society of Surgical Oncology.
Conflict of interest statement
CONFLICT OF INTEREST No potential conflict of interest relevant to this article was reported.
Figures

References
-
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. - PubMed
-
- Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24:2903–9. - PubMed
-
- National Comprehensive Cancer Network (NCCN) NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines 2018) [Internet] Plymouth Meeting (PA): NCCN; c2019. [cited 2020 Dec 29]. Available from: https://www.nccn.org/professionals/physician_gls/default.aspx .
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

