An experimental study: the effect of S. boulardii on abemaciclibinduced diarrhea
- PMID: 36945921
- PMCID: PMC10388128
- DOI: 10.55730/1300-0144.5557
An experimental study: the effect of S. boulardii on abemaciclibinduced diarrhea
Abstract
Background: In our study, we aimed to investigate the protective effects of Saccharomyces boulardii on abemaciclib-induced diarrhea model, which is a commonly used drug in breast cancer.
Methods: Thirty rats were divided into 3 groups as control (Group 1), abemaciclib (Group 2), and abemaciclib + Saccharomyces boulardii (Group 3) groups. The clinical status, body weight, and defecation status were monitored daily. At the end of the 15-day experiment period, the rats were killed with high-dose anesthesia and the resected small intestine segments were evaluated histopathologically. Lesions were classified according to thickening of the villus, inflammation and edema of mucosa and intraepithelial leukocyte accumulation. Then, mean values of both crypt depths and villi thicknesses were calculated for each rat. Normal distribution assumption was controlled with the Shapiro-Wilk test. One-way analysis of variance for normally distributed variables in the comparisons of more than two independent groups and Kruskal-Wallis test for nonnormally distributed variables were used. The significance value was accepted as 0.05.
Results: : There was one death in Group 3, but none in the others. There were no findings of mucositis in Group I. There was mild diarrhea and weight loss in only one rat in Group 1. For the comparison of the severity of diarrhea (72.5%/39%) and weight loss (72.5%/45%), a decrease was found in Group 3 according to Group 2 (p < 0.01). Histopathological findings such as edema, inflammation, and intraepithelial leukocyte accumulation also showed a decrease in Group 3 compared to Group 2 (p < 0.01).
Discussion: Saccharomyces boulardii should be considered as a treatment option in abaemaciclib (chemotherapy)-induced diarrhea. Further comparative studies and in vivo human randomized controlled studies can be conducted in the future.
Keywords: Breast cancer; Saccharomyces boulardii; abemaciclib; diarrhea.
Figures
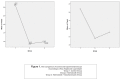
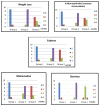

References
-
- Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. The Lancet Oncology. 2015;16(1):25–35. doi: 10.1016/S1470-2045(14)71159-3. - DOI - PubMed
-
- Dickler MN, Tolaney SM, Rugo HS, Cortés J, Diéras V, et al. MONARCH 1, A Phase II Study of Abemaciclib, a CDK4 and CDK6 Inhibitor, as a Single Agent, in Patients with Refractory HR+/HER2− Metastatic Breast Cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2017;23(17):5218–5224. doi: 10.1158/1078-0432.CCR-17-0754. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

