Succinate metabolism and its regulation of host-microbe interactions
- PMID: 36946592
- PMCID: PMC10038034
- DOI: 10.1080/19490976.2023.2190300
Succinate metabolism and its regulation of host-microbe interactions
Abstract
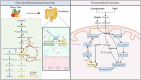
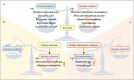
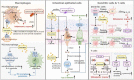
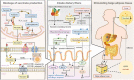
Succinate is a circulating metabolite, and the relationship between abnormal changes in the physiological concentration of succinate and inflammatory diseases caused by the overreaction of certain immune cells has become a research focus. Recent investigations have shown that succinate produced by the gut microbiota has the potential to regulate host homeostasis and treat diseases such as inflammation. Gut microbes are important for maintaining intestinal homeostasis. Microbial metabolites serve as nutrients in energy metabolism, and act as signal molecules that stimulate host cell and organ function and affect the structural balance between symbiotic gut microorganisms. This review focuses on succinate as a metabolite of both host cells and gut microbes and its involvement in regulating the gut - immune tissue axis by activating intestinal mucosal cells, including macrophages, dendritic cells, and intestinal epithelial cells. We also examined its role as the mediator of microbiota - host crosstalk and its potential function in regulating intestinal microbiota homeostasis. This review explores feasible ways to moderate succinate levels and provides new insights into succinate as a potential target for microbial therapeutics for humans.
Keywords: Succinate; gut microbiota; gut–immune tissue axis; immune cells; inflammation.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
