Impact of HPV testing in opportunistic cervical screening: Support for primary HPV screening in the United States
- PMID: 36946690
- PMCID: PMC10639031
- DOI: 10.1002/ijc.34519
Impact of HPV testing in opportunistic cervical screening: Support for primary HPV screening in the United States
Abstract
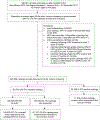
Human papillomavirus (HPV) testing for cervical screening increases diagnosis of precancer and reduces the incidence of cervical cancer more than cytology alone. However, real-world evidence from diverse practice settings is lacking for the United States (U.S.) to support clinician adoption of primary HPV screening. Using a population-based registry, which captures all cervical cytology (with or without HPV testing) and all cervical biopsies, we conducted a real-world evidence study of screening in women aged 30 to 64 years across the entire state of New Mexico. Negative cytology was used to distinguish cotests from reflex HPV tests. A total of 264 198 cervical screening tests (with exclusions based on clinical history) were recorded as the first screening test between 2014 and 2017. Diagnoses of cervical intraepithelial neoplasia grades 2 or 3 or greater (CIN2+, CIN3+) from 2014 to 2019 were the main outcomes. Of cytology-negative screens, 165 595 (67.1%) were cotests and 4.8% of these led to biopsy within 2 years vs 3.2% in the cytology-only group. Among cytology-negative, HPV tested women, 347 of 398 (87.2%) CIN2+ cases were diagnosed in HPV-positive women, as were 147 of 164 (89.6%) CIN3+ cases. Only 29/921 (3.2%) CIN3+ and 67/1964 (3.4%) CIN2+ cases were diagnosed in HPV-negative, cytology-positive women with biopsies. Under U.S. opportunistic screening, across a diversity of health care delivery practices, and in a population suffering multiple disparities, we show adding HPV testing to cytology substantially increased the yield of CIN2+ and CIN3+. CIN3+ was rarely diagnosed in HPV-negative women with abnormal cytology, supporting U.S. primary HPV-only screening.
Keywords: cervical cancer prevention; cervical cancer screening; cotesting vs cytology; opportunistic cervical screening; primary HPV screening.
© 2023 The Authors. International Journal of Cancer published by John Wiley & Sons Ltd on behalf of UICC.
Conflict of interest statement
Conflict of Interest
All other authors have nothing to disclose.
Figures
References
-
- Van Den Brule AJ, Walboomers JM, Du Maine M, Kenemans P, Meijer CJ. Difference in prevalence of human papillomavirus genotypes in cytomorphologically normal cervical smears is associated with a history of cervical intraepithelial neoplasia. Int J Cancer 1991;48: 404–8. - PubMed
-
- Cuzick J, Terry G, Ho L, Hollingworth T, Anderson M. Human papillomavirus type 16 DNA in cervical smears as predictor of high-grade cervical cancer. Lancet 1992;339: 959–60. - PubMed
-
- Cuzick J, Szarewski A, Terry G, Hanby A, Maddox P, Anderson M, Steele SJ, Guillebaud J, Kocjean C. Human papillomavirus testing in primary cervical screening. Lancet 1995;345: 1533–6. - PubMed
-
- Schiffman M, Herrero R, Hildesheim A, Sherman ME, Bratti M, Wacholder S, Alfaro M, Hutchinson M, Morales J, Greenberg MD, Lorincz AT. HPV DNA Testing in Cervical Cancer Screening: Results From Women in a High-Risk Province of Costa Rica. JAMA 2000;283: 87–93. - PubMed
-
- Saslow D, Runowicz CD, Solomon D, Moscicki AB, Smith RA, Eyre HJ, Cohen C. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin 2002;52: 342–62. - PubMed

