Role of the Extracytoplasmic Function Sigma Factor SigE in the Stringent Response of Mycobacterium tuberculosis
- PMID: 36946740
- PMCID: PMC10100808
- DOI: 10.1128/spectrum.02944-22
Role of the Extracytoplasmic Function Sigma Factor SigE in the Stringent Response of Mycobacterium tuberculosis
Abstract
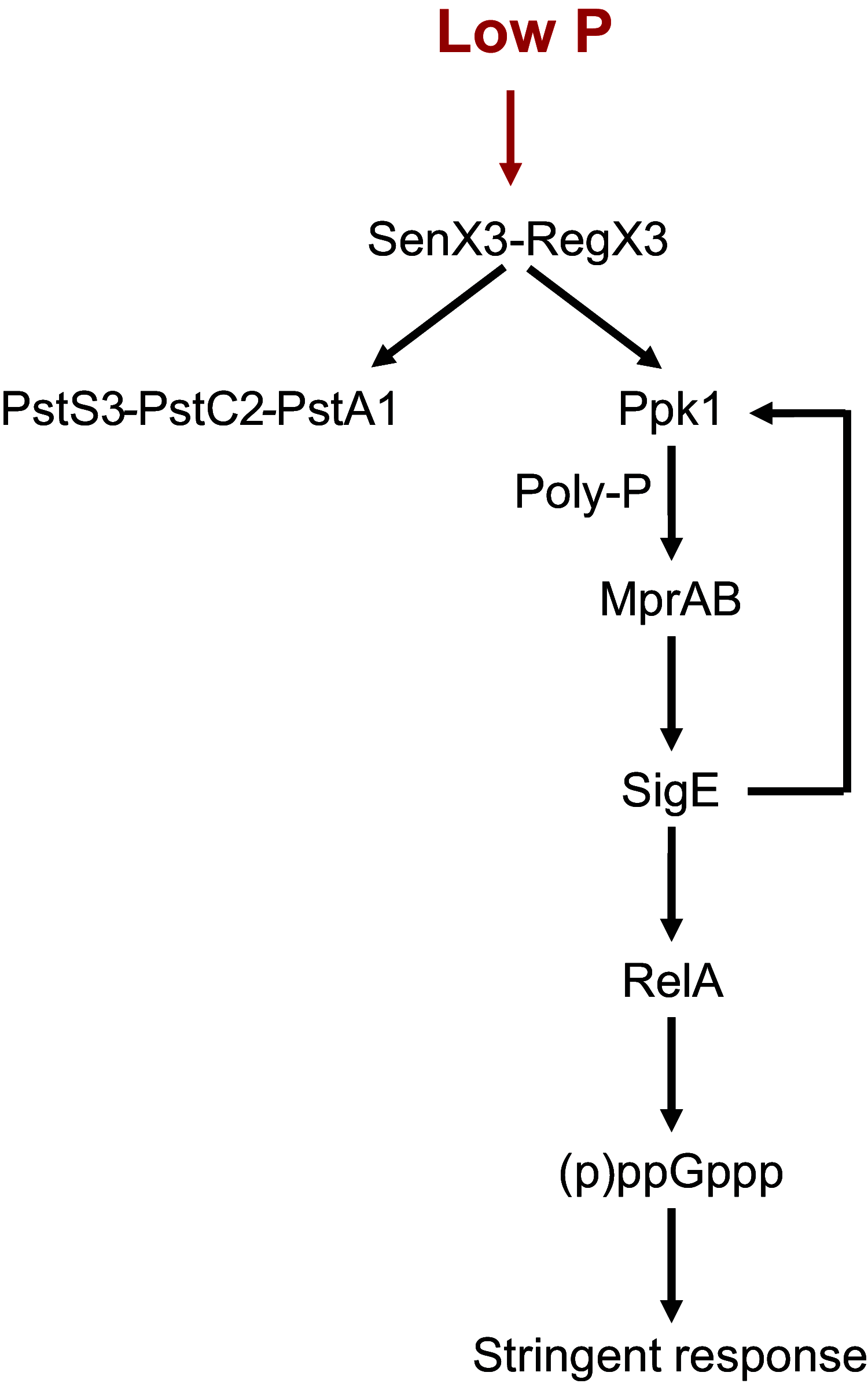
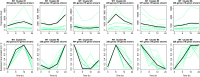
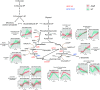
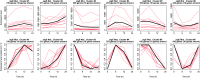
Bacteria respond to nutrient starvation implementing the stringent response, a stress signaling system resulting in metabolic remodeling leading to decreased growth rate and energy requirements. A well-characterized model of stringent response in Mycobacterium tuberculosis is the one induced by growth in low phosphate. The extracytoplasmic function (ECF) sigma factor SigE was previously suggested as having a key role in the activation of stringent response. In this study, we challenge this hypothesis by analyzing the temporal dynamics of the transcriptional response of a sigE mutant and its wild-type parental strain to low phosphate using RNA sequencing. We found that both strains responded to low phosphate with a typical stringent response trait, including the downregulation of genes encoding ribosomal proteins and RNA polymerase. We also observed transcriptional changes that support the occurring of an energetics imbalance, compensated by a reduced activity of the electron transport chain, decreased export of protons, and a remodeling of central metabolism. The most striking difference between the two strains was the induction in the sigE mutant of several stress-related genes, in particular, the genes encoding the ECF sigma factor SigH and the transcriptional regulator WhiB6. Since both proteins respond to redox unbalances, their induction suggests that the sigE mutant is not able to maintain redox homeostasis in response to the energetics imbalance induced by low phosphate. In conclusion, our data suggest that SigE is not directly involved in initiating stringent response but in protecting the cell from stress consequent to the low phosphate exposure and activation of stringent response. IMPORTANCE Mycobacterium tuberculosis can enter a dormant state enabling it to establish latent infections and to become tolerant to antibacterial drugs. Dormant bacteria's physiology and the mechanism(s) used by bacteria to enter dormancy during infection are still unknown due to the lack of reliable animal models. However, several in vitro models, mimicking conditions encountered during infection, can reproduce different aspects of dormancy (growth arrest, metabolic slowdown, drug tolerance). The stringent response, a stress response program enabling bacteria to cope with nutrient starvation, is one of them. In this study, we provide evidence suggesting that the sigma factor SigE is not directly involved in the activation of stringent response as previously hypothesized, but it is important to help the bacteria to handle the metabolic stress related to the adaptation to low phosphate and activation of stringent response, thus giving an important contribution to our understanding of the mechanism behind stringent response development.
Keywords: Mycobacterium tuberculosis; sigma factors; stringent response; transcriptional regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- World Health Organization. 2021. Global tuberculosis report 2021. World Health Organization, Geneva, Switzerland. https://www.who.int/publications/i/item/9789240037021. Retrieved 11 February 2022.
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

