Kinetics of Arf1 inactivation regulates Golgi organisation and function in non-adherent fibroblasts
- PMID: 36946871
- PMCID: PMC10187640
- DOI: 10.1242/bio.059669
Kinetics of Arf1 inactivation regulates Golgi organisation and function in non-adherent fibroblasts
Abstract
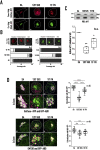
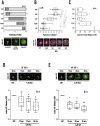
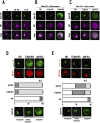
Arf1 belongs to the Arf family of small GTPases that localise at the Golgi and plasma membrane. Active Arf1 plays a crucial role in regulating Golgi organisation and function. In mouse fibroblasts, loss of adhesion triggers a consistent drop (∼50%) in Arf1 activation that causes the Golgi to disorganise but not fragment. In suspended cells, the trans-Golgi (GalTase) disperses more prominently than cis-Golgi (Man II), accompanied by increased active Arf1 (detected using GFP-ABD: ARHGAP10 Arf1 binding domain) associated with the cis-Golgi compartment. Re-adhesion restores Arf1 activation at the trans-Golgi as it reorganises. Arf1 activation at the Golgi is regulated by Arf1 Guanine nucleotide exchange factors (GEFs), GBF1, and BIG1/2. In non-adherent fibroblasts, the cis-medial Golgi provides a unique setting to test and understand the role GEF-mediated Arf1 activation has in regulating Golgi organisation. Labelled with Man II-GFP, non-adherent fibroblasts treated with increasing concentrations of Brefeldin-A (BFA) (which inhibits BIG1/2 and GBF1) or Golgicide A (GCA) (which inhibits GBF1 only) comparably decrease active Arf1 levels. They, however, cause a concentration-dependent increase in cis-medial Golgi fragmentation and fusion with the endoplasmic reticulum (ER). Using selected BFA and GCA concentrations, we find a change in the kinetics of Arf1 inactivation could mediate this by regulating cis-medial Golgi localisation of GBF1. On loss of adhesion, a ∼50% drop in Arf1 activation over 120 min causes the Golgi to disorganise. The kinetics of this drop, when altered by BFA or GCA treatment causes a similar decline in Arf1 activation but over 10 min. This causes the Golgi to now fragment which affects cell surface glycosylation and re-adherent cell spreading. Using non-adherent fibroblasts this study reveals the kinetics of Arf1 inactivation, with active Arf1 levels, to be vital for Golgi organisation and function.
Keywords: Adhesion; Arf1; GBF1; Golgi; Kinetics.
© 2023. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures



Similar articles
-
Dynamics of GBF1, a Brefeldin A-sensitive Arf1 exchange factor at the Golgi.Mol Biol Cell. 2005 Mar;16(3):1213-22. doi: 10.1091/mbc.e04-07-0599. Epub 2004 Dec 22. Mol Biol Cell. 2005. PMID: 15616190 Free PMC article.
-
Modeling the dynamic behaviors of the COPI vesicle formation regulators, the small GTPase Arf1 and its activating Sec7 guanine nucleotide exchange factor GBF1 on Golgi membranes.Mol Biol Cell. 2021 Mar 1;32(5):446-459. doi: 10.1091/mbc.E20-09-0587. Epub 2021 Jan 6. Mol Biol Cell. 2021. PMID: 33405949 Free PMC article.
-
Rab1b-GBF1-ARF1 Secretory Pathway Axis Is Required for Birnavirus Replication.J Virol. 2022 Feb 23;96(4):e0200521. doi: 10.1128/JVI.02005-21. Epub 2021 Dec 8. J Virol. 2022. PMID: 34878889 Free PMC article.
-
Large Arf1 guanine nucleotide exchange factors: evolution, domain structure, and roles in membrane trafficking and human disease.Mol Genet Genomics. 2009 Oct;282(4):329-50. doi: 10.1007/s00438-009-0473-3. Epub 2009 Aug 11. Mol Genet Genomics. 2009. PMID: 19669794 Free PMC article. Review.
-
Regulating the regulators: role of phosphorylation in modulating the function of the GBF1/BIG family of Sec7 ARF-GEFs.FEBS Lett. 2020 Jul;594(14):2213-2226. doi: 10.1002/1873-3468.13798. Epub 2020 May 14. FEBS Lett. 2020. PMID: 32333796 Review.
Cited by
-
A transporter's doom or destiny: SLC6A1 in health and disease, novel molecular targets and emerging therapeutic prospects.Front Mol Neurosci. 2024 Aug 29;17:1466694. doi: 10.3389/fnmol.2024.1466694. eCollection 2024. Front Mol Neurosci. 2024. PMID: 39268250 Free PMC article. Review.
-
ARF1-Related Diseases in China: The Initial Study of Phenotype and Molecular Profile.J Cell Mol Med. 2025 Jul;29(14):e70655. doi: 10.1111/jcmm.70655. J Cell Mol Med. 2025. PMID: 40689678 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

