Safety and Efficacy of Topical Lovastatin Plus Cholesterol Cream vs Topical Lovastatin Cream Alone for the Treatment of Disseminated Superficial Actinic Porokeratosis: A Randomized Clinical Trial
- PMID: 36947042
- PMCID: PMC10034663
- DOI: 10.1001/jamadermatol.2023.0205
Safety and Efficacy of Topical Lovastatin Plus Cholesterol Cream vs Topical Lovastatin Cream Alone for the Treatment of Disseminated Superficial Actinic Porokeratosis: A Randomized Clinical Trial
Abstract
Importance: Disseminated superficial actinic porokeratosis (DSAP) is an inherited or sporadic disorder of keratinization associated with germline variations. There is no effective standard of care therapy for DSAP, but treatment with topical lovastatin combined with cholesterol cream has shown promise.
Objectives: To evaluate and compare the safety and efficacy of topical lovastatin 2% plus cholesterol 2% cream (lovastatin-cholesterol) and topical lovastatin 2% cream (lovastatin) alone in adults diagnosed with DSAP.
Design, setting, and participants: This patient- and assessor-blinded, randomized clinical trial was conducted at the Medical University of South Carolina between August 3, 2020, and April 28, 2021. Nonpregnant adults with a previous clinical or histological diagnosis of DSAP were eligible. Data were blindly analyzed after study completion.
Interventions: Participants were randomized to once- or twice-daily application of either lovastatin-cholesterol cream (n = 17) or lovastatin cream (n = 14) to symptomatic regions for 12 weeks.
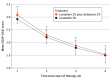
Main outcomes and measures: The primary efficacy measure was the effect of the treatment on DSAP at the end of treatment (12 weeks) as measured by the DSAP General Assessment Severity Index (DSAP-GASI; scored from 0-4, with 0 indicating clear and 4 indicating severe). Treatment efficacy was based on investigator-standardized photographs provided by the participants because of the need for evaluation via telehealth during the COVID-19 pandemic. Secondary efficacy measures included patient-reported outcomes, application frequency, and adverse events (AEs).
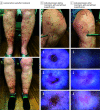
Results: Of the 87 participants screened, 32 were enrolled. One participant randomized to receive lovastatin-cholesterol did not receive the intervention, leaving 17 participants (mean [range] age, 59.2 [40-83] years; 13 females [76.5%]; all White) allocated to receive lovastatin-cholesterol treatment and 14 participants (13 female [92.9%]; mean (range) age, 53.7 [33-71] years; all White) to receive lovastatin treatment. Twelve participants in each treatment group qualified for the analysis. Disease severity decreased from week 1 to week 12 by 50.0% (from 3.08 [95% CI, 2.57-3.60] to 1.54 (95% CI, 1.04-2.05] points on the DSAP-GASI; P < .001) in the lovastatin-cholesterol group and 51.4% (from 2.92 [95% CI, 2.40-3.43] to 1.50 [95% CI, 0.99-2.01] points; P < .001) in the lovastatin group. There was no significant difference between the treatment groups according to application frequency at the end of 12 weeks. Adverse events reported included myalgia (n = 2), elevation in the creatine kinase level (n = 1), application discomfort (n = 4), and rash (n = 1). No serious AEs occurred, and all participants with an AE were able to complete the study.
Conclusions and relevance: This randomized clinical trial found improvements in DSAP severity in both treatment groups, without serious AEs, indicating a limited benefit with the addition of cholesterol. These results suggest that lovastatin cream may be a new primary treatment option for patients diagnosed with DSAP.
Trial registration: ClinicalTrials.gov Identifier: NCT04359823.
Conflict of interest statement
Figures

References
-
- Shakhbazova A, Hinds B, Marsch AF. Lichenoid inflammation of DSAP lesions following treatment with durvalumab, olaparib and paclitaxel: a potential diagnostic pitfall mimicking lichenoid drug eruptions associated with PDL-1 inhibitors. Dermatol Online J. 2020;26(3):13030/qt7nf6c8hc. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

