Covid-19 Surveillance Testing and Resident Outcomes in Nursing Homes
- PMID: 36947467
- PMCID: PMC10052223
- DOI: 10.1056/NEJMoa2210063
Covid-19 Surveillance Testing and Resident Outcomes in Nursing Homes
Abstract
Background: Despite widespread adoption of surveillance testing for coronavirus disease 2019 (Covid-19) among staff members in skilled nursing facilities, evidence is limited regarding its relationship with outcomes among facility residents.
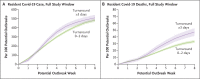
Methods: Using data obtained from 2020 to 2022, we performed a retrospective cohort study of testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) among staff members in 13,424 skilled nursing facilities during three pandemic periods: before vaccine approval, before the B.1.1.529 (omicron) variant wave, and during the omicron wave. We assessed staff testing volumes during weeks without Covid-19 cases relative to other skilled nursing facilities in the same county, along with Covid-19 cases and deaths among residents during potential outbreaks (defined as the occurrence of a case after 2 weeks with no cases). We reported adjusted differences in outcomes between high-testing facilities (90th percentile of test volume) and low-testing facilities (10th percentile). The two primary outcomes were the weekly cumulative number of Covid-19 cases and related deaths among residents during potential outbreaks.
Results: During the overall study period, 519.7 cases of Covid-19 per 100 potential outbreaks were reported among residents of high-testing facilities as compared with 591.2 cases among residents of low-testing facilities (adjusted difference, -71.5; 95% confidence interval [CI], -91.3 to -51.6). During the same period, 42.7 deaths per 100 potential outbreaks occurred in high-testing facilities as compared with 49.8 deaths in low-testing facilities (adjusted difference, -7.1; 95% CI, -11.0 to -3.2). Before vaccine availability, high- and low-testing facilities had 759.9 cases and 1060.2 cases, respectively, per 100 potential outbreaks (adjusted difference, -300.3; 95% CI, -377.1 to -223.5), along with 125.2 and 166.8 deaths (adjusted difference, -41.6; 95% CI, -57.8 to -25.5). Before the omicron wave, the numbers of cases and deaths were similar in high- and low-testing facilities; during the omicron wave, high-testing facilities had fewer cases among residents, but deaths were similar in the two groups.
Conclusions: Greater surveillance testing of staff members at skilled nursing facilities was associated with clinically meaningful reductions in Covid-19 cases and deaths among residents, particularly before vaccine availability.
Copyright © 2023 Massachusetts Medical Society.
Figures
Comment in
-
Covid-19 Surveillance Testing in Nursing Homes.N Engl J Med. 2023 Jun 8;388(23):2207. doi: 10.1056/NEJMc2304781. N Engl J Med. 2023. PMID: 37285543 No abstract available.
-
Covid-19 Surveillance Testing in Nursing Homes. Reply.N Engl J Med. 2023 Jun 8;388(23):2207-2208. doi: 10.1056/NEJMc2304781. N Engl J Med. 2023. PMID: 37285544 No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
