Parasitism causes changes in caterpillar odours and associated bacterial communities with consequences for host-location by a hyperparasitoid
- PMID: 36947551
- PMCID: PMC10069771
- DOI: 10.1371/journal.ppat.1011262
Parasitism causes changes in caterpillar odours and associated bacterial communities with consequences for host-location by a hyperparasitoid
Abstract
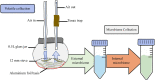
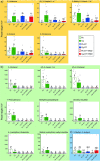
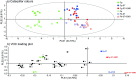
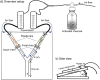
Microorganisms living in and on macroorganisms may produce microbial volatile compounds (mVOCs) that characterise organismal odours. The mVOCs might thereby provide a reliable cue to carnivorous enemies in locating their host or prey. Parasitism by parasitoid wasps might alter the microbiome of their caterpillar host, affecting organismal odours and interactions with insects of higher trophic levels such as hyperparasitoids. Hyperparasitoids parasitise larvae or pupae of parasitoids, which are often concealed or inconspicuous. Odours of parasitised caterpillars aid them to locate their host, but the origin of these odours and its relationship to the caterpillar microbiome are unknown. Here, we analysed the odours and microbiome of the large cabbage white caterpillar Pieris brassicae in relation to parasitism by its endoparasitoid Cotesia glomerata. We identified how bacterial presence in and on the caterpillars is correlated with caterpillar odours and tested the attractiveness of parasitised and unparasitised caterpillars to the hyperparasitoid Baryscapus galactopus. We manipulated the presence of the external microbiome and the transient internal microbiome of caterpillars to identify the microbial origin of odours. We found that parasitism by C. glomerata led to the production of five characteristic volatile products and significantly affected the internal and external microbiome of the caterpillar, which were both found to have a significant correlation with caterpillar odours. The preference of the hyperparasitoid was correlated with the presence of the external microbiome. Likely, the changes in external microbiome and body odour after parasitism were driven by the resident internal microbiome of caterpillars, where the bacterium Wolbachia sp. was only present after parasitism. Micro-injection of Wolbachia in unparasitised caterpillars increased hyperparasitoid attraction to the caterpillars compared to untreated caterpillars, while no differences were found compared to parasitised caterpillars. In conclusion, our results indicate that host-parasite interactions can affect multi-trophic interactions and hyperparasitoid olfaction through alterations of the microbiome.
Copyright: © 2023 Bourne et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Caterpillar-parasitoid interactions: species-specific influences on host microbiome composition.FEMS Microbiol Ecol. 2024 Sep 14;100(10):fiae115. doi: 10.1093/femsec/fiae115. FEMS Microbiol Ecol. 2024. PMID: 39165109 Free PMC article.
-
Body odors of parasitized caterpillars give away the presence of parasitoid larvae to their primary hyperparasitoid enemies.J Chem Ecol. 2014 Sep;40(9):986-95. doi: 10.1007/s10886-014-0500-7. Epub 2014 Sep 19. J Chem Ecol. 2014. PMID: 25236382
-
Parasitism by endoparasitoid wasps alters the internal but not the external microbiome in host caterpillars.Anim Microbiome. 2021 Oct 15;3(1):73. doi: 10.1186/s42523-021-00135-y. Anim Microbiome. 2021. PMID: 34654483 Free PMC article.
-
The Ecology of Hyperparasitoids.Annu Rev Entomol. 2022 Jan 7;67:143-161. doi: 10.1146/annurev-ento-060921-072718. Epub 2021 Oct 4. Annu Rev Entomol. 2022. PMID: 34606363 Review.
-
Attraction of parasitic wasps by caterpillar-damaged plants.Novartis Found Symp. 1999;223:21-32; discussion 32-8. doi: 10.1002/9780470515679.ch3. Novartis Found Symp. 1999. PMID: 10549546 Review.
Cited by
-
Caterpillar-parasitoid interactions: species-specific influences on host microbiome composition.FEMS Microbiol Ecol. 2024 Sep 14;100(10):fiae115. doi: 10.1093/femsec/fiae115. FEMS Microbiol Ecol. 2024. PMID: 39165109 Free PMC article.
-
Parasitoid Calyx Fluid and Venom Affect Bacterial Communities in Their Lepidopteran Host Labial Salivary Glands.Microb Ecol. 2025 Apr 23;88(1):33. doi: 10.1007/s00248-025-02535-y. Microb Ecol. 2025. PMID: 40266381 Free PMC article.
-
Influence of Endogenous Bacteria on Behavioral Responses in Leptocybe invasa: An Analysis of mVOCs.Insects. 2024 Jun 16;15(6):455. doi: 10.3390/insects15060455. Insects. 2024. PMID: 38921169 Free PMC article.
-
Microbiota and the volatile profile of avian nests are associated with each other and with the intensity of parasitism.FEMS Microbiol Ecol. 2024 Sep 14;100(10):fiae106. doi: 10.1093/femsec/fiae106. FEMS Microbiol Ecol. 2024. PMID: 39049462 Free PMC article.
-
Effect of Cotesia ruficrus Parasitization on Diversity and Community Composition of Intestinal Bacteria in Spodoptera frugiperda.Insects. 2024 Jul 27;15(8):570. doi: 10.3390/insects15080570. Insects. 2024. PMID: 39194775 Free PMC article.
References
-
- Margulis L. Symbiogenesis and symbionticism. In: Margulis L, Fester R, editors. Symbiosis as a source of evolutionary innovation: speciation and morphogenesis. Cambridge, MA: MIT Press; 1991. pp. 1–14. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

