Staphylococcus aureus FadB is a dehydrogenase that mediates cholate resistance and survival under human colonic conditions
- PMID: 36947574
- PMCID: PMC10191381
- DOI: 10.1099/mic.0.001314
Staphylococcus aureus FadB is a dehydrogenase that mediates cholate resistance and survival under human colonic conditions
Abstract
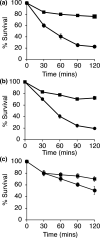
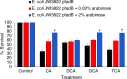
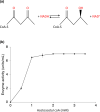
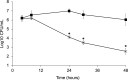
Staphylococcus aureus is a common colonizer of the human gut and in doing so it must be able to resist the actions of the host's innate defences. Bile salts are a class of molecules that possess potent antibacterial activity that control growth. Bacteria that colonize and survive in that niche must be able to resist the action of bile salts, but the mechanisms by which S. aureus does so are poorly understood. Here we show that FadB is a bile-induced oxidoreductase which mediates bile salt resistance and when heterologously expressed in Escherichia coli renders them resistant. Deletion of fadB attenuated survival of S. aureus in a model of the human distal colon.
Keywords: FadB; Staphylococcus aureus; bile acids; cholate; colon; dehydrogenase.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures


References
-
- Acton DS, Tempelmans Plat-Sinnige M, van Wamel W, de Groot N, van Belkum A. Intestinal carriage of Staphylococcus aureus: how does its frequency compare with that of nasal carriage and what is its clinical impact? Eur J Clin Microbiol Infect Dis. 2009;28:115–127. doi: 10.1007/s10096-008-0602-7. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

