Discovery of Nanomolar DCAF1 Small Molecule Ligands
- PMID: 36948210
- PMCID: PMC10108359
- DOI: 10.1021/acs.jmedchem.2c02132
Discovery of Nanomolar DCAF1 Small Molecule Ligands
Abstract
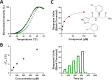
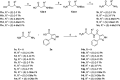
DCAF1 is a substrate receptor of two distinct E3 ligases (CRL4DCAF1 and EDVP), plays a critical physiological role in protein degradation, and is considered a drug target for various cancers. Antagonists of DCAF1 could be used toward the development of therapeutics for cancers and viral treatments. We used the WDR domain of DCAF1 to screen a 114-billion-compound DNA encoded library (DEL) and identified candidate compounds using similarity search and machine learning. This led to the discovery of a compound (Z1391232269) with an SPR KD of 11 μM. Structure-guided hit optimization led to the discovery of OICR-8268 (26e) with an SPR KD of 38 nM and cellular target engagement with EC50 of 10 μM as measured by cellular thermal shift assay (CETSA). OICR-8268 is an excellent tool compound to enable the development of next-generation DCAF1 ligands toward cancer therapeutics, further investigation of DCAF1 functions in cells, and the development of DCAF1-based PROTACs.
Conflict of interest statement
The authors declare the following competing financial interest(s): J.P.G, Y.Z., M.A.G., A.D.K. are employees of X-Chem. M.v.R., J.S.D, B.L.S. and J.W.C. are or were (C.J.M.) employees of Relay Therapeutics and were employees of X-Chem at the time of writing. Employees and past employees may hold stocks and shares. All other authors declare no conflict of interest.
Figures
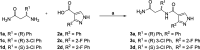


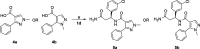




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Medical
Molecular Biology Databases

