Chemical tools for the opioids
- PMID: 36948231
- PMCID: PMC10247539
- DOI: 10.1016/j.mcn.2023.103845
Chemical tools for the opioids
Abstract
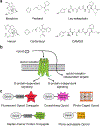
The opioids are potent and widely used pain management medicines despite also possessing severe liabilities that have fueled the opioid crisis. The pharmacological properties of the opioids primarily derive from agonism or antagonism of the opioid receptors, but additional effects may arise from specific compounds, opioid receptors, or independent targets. The study of the opioids, their receptors, and the development of remediation strategies has benefitted from derivatization of the opioids as chemical tools. While these studies have primarily focused on the opioids in the context of the opioid receptors, these chemical tools may also play a role in delineating mechanisms that are independent of the opioid receptors. In this review, we describe recent advances in the development and applications of opioid derivatives as chemical tools and highlight opportunities for the future.
Keywords: Adjuvants; Binding affinity; Bioconjugation; Chemical probe; Fluorescence; Hapten; Immunomodulator; Immunotherapeutic; Opioid; Opioid probe; Opioid receptor; Opioid use disorder; Photo-affinity labeling; Photocage; Photopharmacology; Photoswitch; Small molecule; Spatiotemporal control; Vaccine.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare no conflicts of interest.
Figures

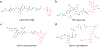
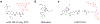
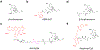
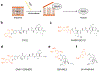


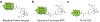

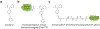
References
-
- Martin WR, Eades CG, Thompson JA, Huppler RE & Gilbert PE The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J. Pharmacol. Exp. Ther 197, 517–532 (1976). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

