Biologic Versus Small Molecule Therapy for Treating Moderate to Severe Atopic Dermatitis: Clinical Considerations
- PMID: 36948491
- PMCID: PMC10164714
- DOI: 10.1016/j.jaip.2023.03.011
Biologic Versus Small Molecule Therapy for Treating Moderate to Severe Atopic Dermatitis: Clinical Considerations
Abstract
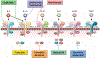
The U.S. Food and Drug Administration approval of dupilumab for moderate-to-severe atopic dermatitis shifted the paradigm from use of broad, systemic immunosuppressants to a safer, targeted treatment and led to the emergence of newer interleukin (IL)-4/IL-13 directed biologics and small molecule therapies, namely Janus kinase (JAK) inhibitors (JAKi). Tralokinumab and emerging (not yet approved) lebrikizumab, which both target IL-13, are alternative biologics to dupilumab. The emerging anti-IL-31 receptor nemolizumab is likely to be used second-line to other biologics, primarily for pruritus. Three JAKi are currently in use for treating atopic dermatitis, 2 of which, abrocitinib and upadacitinib, are U.S. Food and Drug Administration-approved. This review provides an in-depth, practical discussion on use of these biologics and JAKi that are approved or have completed phase 3 clinical trials in pediatric patients and adults, comparing the groups of medications based on available efficacy and safety data.
Keywords: Abrocitinib; Atopic dermatitis; Baricitinib; Biologics; Cytokine signaling; Dupilumab; Eczema; Interleukin-13; Interleukin-4; Janus kinase itnhibitor; Lebrikizumab; Nemolizumab; Tralokinumab; Upadacitinib.
Copyright © 2023 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Dr. Sneha Butala and Rishi Seshadri have no conflicts of interest to report.
Dr. Leslie Castelo-Soccio has previously received a consultant fee in March 2021 for alopecia areata and funding in support of an autoimmune fellowship from in September 2020, both from Pfizer. She serves on the medical advisory board for the National Alopecia Areata Foundation.
Dr. Eric L. Simpson reports grants and/or personal fees from AbbVie, Boehringer Ingelheim, Celgene, Dermavant, Dermira, Eli Lilly, FortéBio, Galderma, Incyte, Kyowa Kirin, LEO Pharma, MedImmune, Menlo Therapeutics, Merck, Novartis, Ortho Dermatologics, Pfizer, Pierre Fabre Dermo Cosmetique, Regeneron, Sanofi, Tioga, and Valeant.
Dr. John J. O’Shea and the National Institutes of Health hold patents related to therapeutic targeting of JAKs, for which Dr. O’Shea also receives royalties.
Dr. Thomas Bieber was speaker and/or consultant and/or Investigator for AbbVie, Affibody, Almirall, AnaptysBio, Arena, Asana Biosciences, ASLAN pharma, Bayer Health, BioVerSys, Böhringer-Ingelheim, Bristol-Myers Squibb, Connect Pharma, Dermavant, DIECE Therapeutics, Domain Therapeutics, EQRx, Galderma, Glenmark, GSK, Incyte, Innovaderm, IQVIA, Janssen, Kirin, Kymab, LEO, LG Chem, Lilly, LÓréal, MSD, Novartis, Numab, OM-Pharma, Pfizer, Pierre Fabre, Q32bio, RAPT, Sanofi/Regeneron, UCB. He is founder and chairman of the board of the non-profit biotech “Davos Biosciences”.
Dr. Amy S. Paller has been an investigator for AbbVie, Dermavant, Eli Lilly, Incyte, Janssen, Krystal, and UCB; a consultant for Aegerion Pharma, Azitra, BioCryst, Boehringer-Ingelheim, Bristol Myers Squibb, Castle Creek, Eli Lilly, Janssen, Krystal, LEO Pharma, Novartis, Regeneron, Sanofi/Genzyme, Seanergy, TWI Biotechnology, and UCB, on the data safety monitoring board for AbbVie, Abeona, Catawba, Galderma, and InMed.
Figures
References
-
- Silverberg JI, Barbarot S, Gadkari A, Simpson EL, Weidinger S, Mina-Osorio P, et al. Atopic dermatitis in the pediatric population: A cross-sectional, international epidemiologic study. Ann Allergy Asthma Immunol. 2021;126(4):417–28 e2. - PubMed
-
- Silverberg JI. Comorbidities and the impact of atopic dermatitis. Ann Allergy Asthma Immunol. 2019;123(2):144–51. - PubMed
-
- Regeneron. DUPIXENT® (dupilumab) injection, for subcutaneous use. Tarrytown, NY. 2022.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

