First-in-human, phase 1 study of PF-06753512, a vaccine-based immunotherapy regimen (VBIR), in non-metastatic hormone-sensitive biochemical recurrence and metastatic castration-resistant prostate cancer (mCRPC)
- PMID: 36948505
- PMCID: PMC10040068
- DOI: 10.1136/jitc-2022-005702
First-in-human, phase 1 study of PF-06753512, a vaccine-based immunotherapy regimen (VBIR), in non-metastatic hormone-sensitive biochemical recurrence and metastatic castration-resistant prostate cancer (mCRPC)
Abstract
Background: This phase 1 study evaluated PF-06753512, a vaccine-based immunotherapy regimen (PrCa VBIR), in two clinical states of prostate cancer (PC), metastatic castration-resistant PC (mCRPC) and biochemical recurrence (BCR).
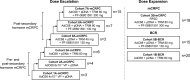
Methods: For dose escalation, patients with mCRPC received intramuscular PrCa VBIR (adenovirus vector and plasmid DNA expressing prostate-specific membrane antigen (PSMA), prostate-specific antigen (PSA), and prostate stem cell antigen (PSCA)) with or without immune checkpoint inhibitors (ICIs, tremelimumab 40 or 80 mg with or without sasanlimab 130 or 300 mg, both subcutaneous). For dose expansion, patients with mCRPC received recommended phase 2 dose (RP2D) of PrCa VBIR plus tremelimumab 80 mg and sasanlimab 300 mg; patients with BCR received PrCa VBIR plus tremelimumab 80 mg (Cohort 1B-BCR) or tremelimumab 80 mg plus sasanlimab 130 mg (Cohort 5B-BCR) without androgen deprivation therapy (ADT). The primary endpoint was safety.
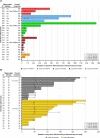
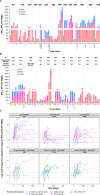
Results: Ninety-one patients were treated in dose escalation (mCRPC=38) and expansion (BCR=35, mCRPC=18). Overall, treatment-related and immune-related adverse events occurred in 64 (70.3%) and 39 (42.9%) patients, with fatigue (40.7%), influenza-like illness (30.8%), diarrhea (23.1%), and immune-related thyroid dysfunction (19.8%) and rash (15.4%), as the most common. In patients with mCRPC, the objective response rate (ORR, 95% CI) was 5.6% (1.2% to 15.4%) and the median radiographic progression-free survival (rPFS) was 5.6 (3.5 to not estimable) months for all; the ORR was 16.7% (3.6% to 41.4%) and 6-month rPFS rate was 45.5% (24.9% to 64.1%) for those who received RP2D with measurable disease (n=18). 7.4% of patients with mCRPC achieved a ≥50% decline in baseline PSA (PSA-50), with a median duration of 4.6 (1.2-45.2) months. In patients with BCR, 9 (25.7%) achieved PSA-50; the median duration of PSA response was 3.9 (1.9-4.2) and 10.1 (6.9-28.8) months for Cohorts 5B-BCR and 1B-BCR. Overall, antigen specific T-cell response was 88.0% to PSMA, 84.0% to PSA, and 80.0% to PSCA.
Conclusions: PrCa VBIR overall demonstrated safety signals similar to other ICI combination trials; significant side effects were seen in some patients with BCR. It stimulated antigen-specific immunity across all cohorts and resulted in modest antitumor activity in patients with BCR without using ADT.
Trial registration number: NCT02616185.
Keywords: immunogenicity, vaccine; prostatic neoplasms; therapies, Investigational; vaccination.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: This work was sponsored by Pfizer. KAA: PI/research funding to institution: Amgen, AstraZeneca, CytomX, Eli Lilly and Company, GlaxoSmithKline, Parker Institute for Cancer Immunotherapy, Pfizer, Trishula. CSH: consulting fees: Astellas, AstraZeneca, Bayer, Blue Earth Diagnostics, Carrick Therapeutics, Clovis Oncology, Ferring Pharmaceuticals, Genentech, Hinova, Janssen, Menarini, Merck Sharp & Dohme, Myovant Sciences, Novartis, Pfizer; contracted research support: Aragon Pharmaceuticals, Astellas, AstraZeneca, Bayer, Clovis Oncology, eFFECTOR Therapeutics, Emergent BioSolutions, Ferring Pharmaceuticals, Medivation, Roche; ownership interest: CTI BioPharma; honoraria: Astellas. LN and X-HZ: nothing to disclose. LJA: consulting or advisory role: AADi; research funding (institution as recipient): Amgen, Astellas, Aveo, Bayer, BioNTech, Bristol Myers Squibb, Calithera Biosciences, Eisai, Eli Lilly and Company, Epizyme, Exelixis, Genentech/Roche, Inovio, Merck, Novartis, Peloton Therapeutics, Pfizer, Seattle Genetics, Surface Oncology; other relationship: Pfizer; uncompensated relationships: Exelixis. TZ: PI/research funding: AbbVie/StemCentrx, Acerta, Astellas, AstraZeneca, Eli Lilly and Company, Janssen, Merck, Merrimack, Mirati Therapeutics, Novartis, OmniSeq, Personal Genome Diagnostics, Pfizer, Regeneron; speaker: Genomic Health (end 2020), Sanofi-Aventis (end 2020); advisory board/consultant: Amgen, Aptitude Health, AstraZeneca, Aveo, Bayer, Bristol Myers Squibb, Calithera Biosciences, Clinical Care Options, Dendreon, Eisai, Eli Lilly and Company, Exelixis, Janssen, Merck, MJH Associates, Peerview, Pfizer, Pharmacyclics, QED Therapeutics, Sanofi-Aventis, Seattle Genetics, Vaniam; employee/stockholder: Archimmune Therapeutics (spouse), Capio Biosciences (spouse), Nanorobotics (spouse). HB: consultancy: Caris, Idera, Myovant, Novocure, Tracon; speaker’s bureau: Guardant360. NJV: PI/research funding: none; advisory boards: Caris, Clovis, Eisai, Fujifilm, Gilead Sciences, Merck, OnQuality Pharmaceuticals, Pfizer-Myovant, Sanofi-Aventis, Seagen; speaker’s bureau: Aveo, Bayer, Caris, Janssen, PER, Pfizer-Myovant, Sanofi, Seagen. SMP: PI funding: Janssen, Merck, Pfizer, UroGen Pharma. MTS: paid consultant and/or received honoraria: AstraZeneca, PharmaIn, Resverlogix, Sanofi; research funding to institution: Ambrx, AstraZeneca, Bristol Myers Squibb, F. Hoffman-La Roche, Immunomedics, Janssen, Madison Vaccines, Merck, Pfizer, SignalOne Bio, Tmunity, Zenith Epigenetics. RAM: research funding: Bayer. SB, NC, OB, RL, MK, I-MW, JZ, S-YT, and KAK: employees of Pfizer with stock and/or stock options. KC, HC, and RH: employees of Pfizer at the time of the study. DPP: stock and other ownership interests: Bellicum Pharmaceuticals, TYME Technologies; consulting or advisory role: Advanced Accelerator Applications, Amgen, Astellas, AstraZeneca, Bayer, Bicycle Therapeutics, Boehringer Ingelheim, Bristol Myers Squibb, Clovis Oncology, Eli Lilly and Company, Exelixis, Gilead Sciences, Incyte, Ipsen, Janssen, Mirati Therapeutics, Monopteros Therapeutics, Pfizer, Pharmacyclics, Regeneron, Roche, Seattle Genetics, UroGen Pharma; research funding: Advanced Accelerator Applications (Inst), Agensys (Inst), Astellas Medivation (Inst), AstraZeneca (Inst), Bayer (Inst), BioXcel Therapeutics (Inst), Bristol Myers Squibb (Inst), Clovis Oncology (Inst), Eisai (Inst), Endocyte (Inst), Eli Lilly and Company (Inst), Genentech (Inst), Gilead Sciences (Inst), Innocrin Pharma (Inst), MedImmune (Inst), Medivation (Inst), Merck (Inst), Mirati Therapeutics (Inst), Novartis (Inst), Pfizer (Inst), Progenics (Inst), Replimune (Inst), Roche (Inst), Sanofi (Inst), Seattle Genetics (Inst); expert testimony: Celgene, Sanofi.
Figures
References
-
- U.S. Food and Drug Administration . FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication. Available: www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accele... [Accessed Feb 2023].
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
