Contralateral Afferent Input to Lumbar Lamina I Neurons as a Neural Substrate for Mirror-Image Pain
- PMID: 36948583
- PMCID: PMC10162462
- DOI: 10.1523/JNEUROSCI.1897-22.2023
Contralateral Afferent Input to Lumbar Lamina I Neurons as a Neural Substrate for Mirror-Image Pain
Abstract
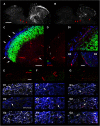
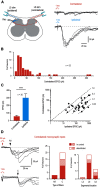
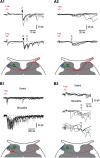
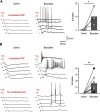
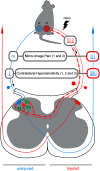
Mirror-image pain arises from pathologic alterations in the nociceptive processing network that controls functional lateralization of the primary afferent input. Although a number of clinical syndromes related to dysfunction of the lumbar afferent system are associated with the mirror-image pain, its morphophysiological substrate and mechanism of induction remain poorly understood. Therefore, we used ex vivo spinal cord preparation of young rats of both sexes to study organization and processing of the contralateral afferent input to the neurons in the major spinal nociceptive projection area Lamina I. We show that decussating primary afferent branches reach contralateral Lamina I, where 27% of neurons, including projection neurons, receive monosynaptic and/or polysynaptic excitatory drive from the contralateral Aδ-fibers and C-fibers. All these neurons also received ipsilateral input, implying their involvement in the bilateral information processing. Our data further show that the contralateral Aδ-fiber and C-fiber input is under diverse forms of inhibitory control. Attenuation of the afferent-driven presynaptic inhibition and/or disinhibition of the dorsal horn network increased the contralateral excitatory drive to Lamina I neurons and its ability to evoke action potentials. Furthermore, the contralateral Aβδ-fibers presynaptically control ipsilateral C-fiber input to Lamina I neurons. Thus, these results show that some lumbar Lamina I neurons are wired to the contralateral afferent system whose input, under normal conditions, is subject to inhibitory control. A pathologic disinhibition of the decussating pathways can open a gate controlling contralateral information flow to the nociceptive projection neurons and, thus, contribute to induction of hypersensitivity and mirror-image pain.SIGNIFICANCE STATEMENT We show that contralateral Aδ-afferents and C-afferents supply lumbar Lamina I neurons. The contralateral input is under diverse forms of inhibitory control and itself controls the ipsilateral input. Disinhibition of decussating pathways increases nociceptive drive to Lamina I neurons and may cause induction of contralateral hypersensitivity and mirror-image pain.
Keywords: dorsal horn; dorsal root potentials; marginal zone; nociception; presynaptic inhibition; primary afferents.
Copyright © 2023 the authors.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
