Retrospective Pediatric Cohort Study Validates NEOS Score and Demonstrates Applicability in Children With Anti-NMDAR Encephalitis
- PMID: 36948591
- PMCID: PMC10032577
- DOI: 10.1212/NXI.0000000000200102
Retrospective Pediatric Cohort Study Validates NEOS Score and Demonstrates Applicability in Children With Anti-NMDAR Encephalitis
Abstract
Background and objectives: Anti-N-methyl-D-aspartate receptor encephalitis (NMDARE) is the most common form of autoimmune encephalitis in children and adults. Although our understanding of the disease mechanisms has progressed, little is known about estimating patient outcomes. Therefore, the NEOS (anti-NMDAR Encephalitis One-Year Functional Status) score was introduced as a tool to predict disease progression in NMDARE. Developed in a mixed-age cohort, it currently remains unclear whether NEOS can be optimized for pediatric NMDARE.
Methods: This retrospective observational study aimed to validate NEOS in a large pediatric-only cohort of 59 patients (median age of 8 years). We reconstructed the original score, adapted it, evaluated additional variables, and assessed its predictive power (median follow-up of 20 months). Generalized linear regression models were used to examine predictability of binary outcomes based on the modified Rankin Scale (mRS). In addition, neuropsychological test results were investigated as alternative cognitive outcome.
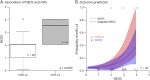
Results: The NEOS score reliably predicted poor clinical outcome (mRS ≥3) in children in the first year after diagnosis (p = 0.0014) and beyond (p = 0.036, 16 months after diagnosis). A score adapted to the pediatric cohort by adjusting the cutoffs of the 5 NEOS components did not improve predictive power. In addition to these 5 variables, further patient characteristics such as the "Herpes simplex virus encephalitis (HSE) status" and "age at disease onset" influenced predictability and could potentially be useful to define risk groups. NEOS also predicted cognitive outcome with higher scores associated with deficits of executive function (p = 0.048) and memory (p = 0.043).
Discussion: Our data support the applicability of the NEOS score in children with NMDARE. Although not yet validated in prospective studies, NEOS also predicted cognitive impairment in our cohort. Consequently, the score could help identify patients at risk of poor overall clinical outcome and poor cognitive outcome and thus aid in selecting not only optimized initial therapies for these patients but also cognitive rehabilitation to improve long-term outcomes.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Conflict of interest statement
The authors report no relevant disclosures. Go to
Figures

References
-
- Mikasova L, Rossi PD, Bouchet D, et al. Disrupted surface cross-talk between NMDA and Ephrin-B2 receptors in anti-NMDA encephalitis. Brain. 2012;135:1606-1621. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
