Protein labeling and crosslinking by covalent aptamers
- PMID: 36948709
- PMCID: PMC10725707
- DOI: 10.1016/bs.mie.2022.08.053
Protein labeling and crosslinking by covalent aptamers
Abstract
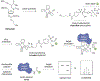
In this chapter, a new approach to the selective modification of native proteins is discussed, using electrophilic covalent aptamers. These biochemical tools are generated through the site-specific incorporation of a label-transferring or crosslinking electrophile into a DNA aptamer. Covalent aptamers provide the ability to transfer a variety of functional handles to a protein of interest or to irreversibly crosslink to the target. Methods for the aptamer-mediated labeling and crosslinking of thrombin are described. Thrombin labeling is fast and selective, in both simple buffer and in human plasma and outcompetes nuclease-mediated degradation. This approach provides facile, sensitive detection of labeled protein by western blot, SDS-PAGE, and mass spectrometry.
Keywords: Aptamer; Electrophile; Nucleic acid; Protein bioconjugation; Protein labeling.
Copyright © 2023 Elsevier Inc. All rights reserved.
Figures
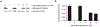


References
-
- Graham D, Parkinson AJ, & Brown T (1998). DNA duplexes stabilized by modified monomer residues: Synthesis and stability. Journal of the Chemical Society, Perkin Transactions, 1(6), 1131–1138. 10.1039/A707031D. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

