Androgens show sex-dependent differences in myelination in immune and non-immune murine models of CNS demyelination
- PMID: 36949062
- PMCID: PMC10033728
- DOI: 10.1038/s41467-023-36846-w
Androgens show sex-dependent differences in myelination in immune and non-immune murine models of CNS demyelination
Abstract
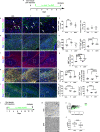
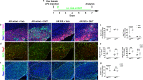
Neuroprotective, anti-inflammatory, and remyelinating properties of androgens are well-characterized in demyelinated male mice and men suffering from multiple sclerosis. However, androgen effects mediated by the androgen receptor (AR), have been only poorly studied in females who make low androgen levels. Here, we show a predominant microglial AR expression in demyelinated lesions from female mice and women with multiple sclerosis, but virtually undetectable AR expression in lesions from male animals and men with multiple sclerosis. In female mice, androgens and estrogens act in a synergistic way while androgens drive microglia response towards regeneration. Transcriptomic comparisons of demyelinated mouse spinal cords indicate that, regardless of the sex, androgens up-regulate genes related to neuronal function integrity and myelin production. Depending on the sex, androgens down-regulate genes related to the immune system in females and lipid catabolism in males. Thus, androgens are required for proper myelin regeneration in females and therapeutic approaches of demyelinating diseases need to consider male-female differences.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures








Similar articles
-
The neural androgen receptor: a therapeutic target for myelin repair in chronic demyelination.Brain. 2013 Jan;136(Pt 1):132-46. doi: 10.1093/brain/aws284. Brain. 2013. PMID: 23365095 Free PMC article.
-
Cortical neuronal densities and cerebral white matter demyelination in multiple sclerosis: a retrospective study.Lancet Neurol. 2018 Oct;17(10):870-884. doi: 10.1016/S1474-4422(18)30245-X. Epub 2018 Aug 22. Lancet Neurol. 2018. PMID: 30143361 Free PMC article.
-
Reduced inflammation accompanies diminished myelin damage and repair in the NG2 null mouse spinal cord.J Neuroinflammation. 2011 Nov 13;8:158. doi: 10.1186/1742-2094-8-158. J Neuroinflammation. 2011. PMID: 22078261 Free PMC article.
-
Neuronal injury in chronic CNS inflammation.Best Pract Res Clin Anaesthesiol. 2010 Dec;24(4):551-62. doi: 10.1016/j.bpa.2010.11.001. Epub 2010 Nov 29. Best Pract Res Clin Anaesthesiol. 2010. PMID: 21619866 Review.
-
Immune promotion of central nervous system remyelination.Prog Brain Res. 1994;103:343-55. doi: 10.1016/s0079-6123(08)61148-6. Prog Brain Res. 1994. PMID: 7886217 Review.
Cited by
-
Differential Effect of Chronic Morphine on Neuronal Degeneration in Male vs. Female Mice.Pathophysiology. 2024 Mar 6;31(1):152-165. doi: 10.3390/pathophysiology31010012. Pathophysiology. 2024. PMID: 38535622 Free PMC article.
-
Crosstalk between androgen signaling and the chemokine receptor CXCR4: a novel strategy to promote myelin regeneration.Neural Regen Res. 2025 Sep 1;20(9):2581-2582. doi: 10.4103/NRR.NRR-D-24-00439. Epub 2024 Sep 24. Neural Regen Res. 2025. PMID: 39503422 Free PMC article. No abstract available.
-
Pharmacogenomic screening identifies and repurposes leucovorin and dyclonine as pro-oligodendrogenic compounds in brain repair.Nat Commun. 2024 Nov 13;15(1):9837. doi: 10.1038/s41467-024-54003-9. Nat Commun. 2024. PMID: 39537633 Free PMC article.
-
The progress in epidemiological, diagnosis and treatment of primary hemifacial spasm.Heliyon. 2024 Sep 30;10(19):e38600. doi: 10.1016/j.heliyon.2024.e38600. eCollection 2024 Oct 15. Heliyon. 2024. PMID: 39430510 Free PMC article. Review.
-
Gender-Specific Fine Motor Skill Learning Is Impaired by Myelin-Targeted Neurofibromatosis Type 1 Gene Mutation.Cancers (Basel). 2024 Jan 23;16(3):477. doi: 10.3390/cancers16030477. Cancers (Basel). 2024. PMID: 38339230 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

