Epigenetic state determines the in vivo efficacy of STING agonist therapy
- PMID: 36949064
- PMCID: PMC10033671
- DOI: 10.1038/s41467-023-37217-1
Epigenetic state determines the in vivo efficacy of STING agonist therapy
Abstract
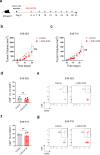
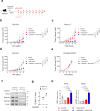
While STING-activating agents have shown limited efficacy in early-phase clinical trials, multiple lines of evidence suggest the importance of tumor cell-intrinsic STING function in mediating antitumor immune responses. Although STING signaling is impaired in human melanoma, its restoration through epigenetic reprogramming can augment its antigenicity and T cell recognition. In this study, we show that reversal of methylation silencing of STING in murine melanoma cell lines using a clinically available DNA methylation inhibitor can improve agonist-induced STING activation and type-I IFN induction, which, in tumor-bearing mice, can induce tumor regression through a CD8+ T cell-dependent immune response. These findings not only provide mechanistic insight into how STING signaling dysfunction in tumor cells can contribute to impaired responses to STING agonist therapy, but also suggest that pharmacological restoration of STING signaling through epigenetic reprogramming might improve the therapeutic efficacy of STING agonists.
© 2023. The Author(s).
Conflict of interest statement
J.J.M. is Associate Center Director at Moffitt Cancer Center, has ownership interest in Aleta Biotherapeutics, CG Oncology, Turnstone Biologics, Ankyra Therapeutics, and AffyImmune Therapeutics, and is a paid consultant/paid advisory board member for ONCoPEP, CG Oncology, Mersana Therapeutics, Turnstone Biologics, Vault Pharma, Ankyra Therapeutics, AffyImmune Therapeutics, UbiVac, Vycellix, and Aleta Biotherapeutics.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

