Enhancing a SARS-CoV-2 nucleocapsid antigen test sensitivity with cost efficient strategy through a cotton intermembrane insertion
- PMID: 36949174
- PMCID: PMC10031715
- DOI: 10.1038/s41598-023-31641-5
Enhancing a SARS-CoV-2 nucleocapsid antigen test sensitivity with cost efficient strategy through a cotton intermembrane insertion
Abstract
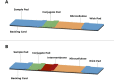
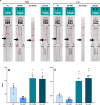
Lateral flow antigen tests have been widely used in the Covid-19 pandemic, allowing faster diagnostic test results and preventing further viral spread through isolation of infected individuals. Accomplishment of this screening must be performed with tests that show satisfactory sensitivity in order to successfully detect the target protein and avoid false negatives. The aim of this study was to create a lateral flow test that could detect SARS-CoV-2 nucleocapsid protein in low concentrations that were comparable to the limits of detection claimed by existing tests from the market. To do so, several adjustments were necessary during research and development of the prototypes until they were consistent with these criteria. The proposed alternatives of increasing the test line antibody concentration and addition of an intermembrane between the conjugate pad and the nitrocellulose membrane were able to increase the sensitivity four-fold and generate a new rapid test prototype called "lateral flow intermembrane immunoassay test" (LFIIT). This prototype showed an adequate limit of detection (2.0 ng mL-1) while maintaining affordability and simplicity in manufacturing processes.
© 2023. The Author(s).
Conflict of interest statement
The authors declare the following financial interests/personal relationships that may be considered as potential competing interests. Marcus Vinícius Mazega Figueredo is the CEO at Hilab. Sérgio Renato Rogal Júnior is the CTO at Hilab. Bernardo Montesanti Machado de Almeida is the CMO at Hilab. Diego Rinaldi Pavesi Nicollete is the R&D in laboratory innovation manager at Hilab, and does not declare any competing interest. Rafael Benedetti is health researcher at Hilab, and does not declare any competing interest. Keyla Kaori Kuniyoshi is health researcher at Hilab,and does not declare any competing interest. Thainá Caroline Schuartz de Jesus is health researcher at Hilab, and does not declare any competing interest. Ava Gevaerd is a researcher in charge of Electrochemistry methods development at Hilab, and does not declare any competing interest. Beatriz Arruda Valença is a former health researcher at Hilab, and does not declare any competing interest. Erika Bergamo Santiago is a former R&D in laboratory innovation manager at Hilab, and does not declare any competing interest.
Figures




Similar articles
-
Comparison of a Prototype SARS-CoV-2 Lateral Flow IMMUNOASSAY with the BinaxNOWTM COVID-19 Antigen CARD.Viruses. 2022 Nov 23;14(12):2609. doi: 10.3390/v14122609. Viruses. 2022. PMID: 36560613 Free PMC article.
-
Fluorescent nanodiamond-based spin-enhanced lateral flow immunoassay for detection of SARS-CoV-2 nucleocapsid protein and spike protein from different variants.Anal Chim Acta. 2022 Oct 16;1230:340389. doi: 10.1016/j.aca.2022.340389. Epub 2022 Sep 14. Anal Chim Acta. 2022. PMID: 36192062 Free PMC article.
-
Absorption-Modulated SiO2@Au Core-Satellite Nanoparticles for Highly Sensitive Detection of SARS-CoV-2 Nucleocapsid Protein in Lateral Flow Immunosensors.ACS Appl Mater Interfaces. 2022 Oct 12;14(40):45189-45200. doi: 10.1021/acsami.2c13303. Epub 2022 Oct 3. ACS Appl Mater Interfaces. 2022. PMID: 36191048
-
Current status of the lateral flow immunoassay for the detection of SARS-CoV-2 in nasopharyngeal swabs.Biochem Med (Zagreb). 2021 Jun 15;31(2):020601. doi: 10.11613/BM.2021.020601. Biochem Med (Zagreb). 2021. PMID: 34140830 Free PMC article. Review.
-
Immunologic Testing for SARS-CoV-2 Infection from the Antigen Perspective.J Clin Microbiol. 2021 Apr 20;59(5):e02160-20. doi: 10.1128/JCM.02160-20. Print 2021 Apr 20. J Clin Microbiol. 2021. PMID: 33318065 Free PMC article. Review.
Cited by
-
Utilizing COVID-19 as a Model for Diagnostics Using an Electrochemical Sensor.Sensors (Basel). 2024 Jun 10;24(12):3772. doi: 10.3390/s24123772. Sensors (Basel). 2024. PMID: 38931556 Free PMC article.
-
Advancements in Cortisol Detection: From Conventional Methods to Next-Generation Technologies for Enhanced Hormone Monitoring.ACS Sens. 2024 Apr 26;9(4):1666-1681. doi: 10.1021/acssensors.3c01912. Epub 2024 Mar 29. ACS Sens. 2024. PMID: 38551608 Free PMC article. Review.
-
Clinical Validation of a Colorimetric Loop-Mediated Isothermal Amplification Using a Portable Device for the Rapid Detection of SARS-CoV-2.Diagnostics (Basel). 2023 Apr 6;13(7):1355. doi: 10.3390/diagnostics13071355. Diagnostics (Basel). 2023. PMID: 37046573 Free PMC article.
-
Optical lateral flow assays in early diagnosis of SARS-CoV-2 infection.Anal Sci. 2024 Sep;40(9):1571-1591. doi: 10.1007/s44211-024-00596-6. Epub 2024 May 17. Anal Sci. 2024. PMID: 38758251 Review.
-
A sensitive gold nanoflower-based lateral flow assay coupled with gold staining technique for the detection of SARS-CoV-2 antigen.Mikrochim Acta. 2024 Jun 29;191(7):434. doi: 10.1007/s00604-024-06502-1. Mikrochim Acta. 2024. PMID: 38951317
References
-
- Holmes KV. Coronaviruses (Coronaviridae) Encyclop. Virol. 1999;1:291–298. doi: 10.1006/rwvi.1999.0055. - DOI
-
- Zaki, A., Van Boheemen, S., Bestebroer, T.M., Osterhaus, A.D., & Fouchier, R.A. Isolation of a Novel Coronavirus from a Man with Pneumonia in Saudi Arabia. N. Engl. J. Med.367, 1814–1820 (2012). - PubMed
-
- Drosten C, Günther S, Preiser W, van der Werf S, Brodt HR, Becker S, Rabenau H, Panning M, Kolesnikova L, Fouchier RA, Berger A, Burguière AM, Cinatl J, Eickmann M, Escriou N, Grywna K, Kramme S, Manuguerra JC, Müller S, Rickerts V, Stürmer M, Vieth S, Klenk HD, Osterhaus AD, Schmitz H, Doerr HW. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

