From primordial clocks to circadian oscillators
- PMID: 36949197
- PMCID: PMC10076222
- DOI: 10.1038/s41586-023-05836-9
From primordial clocks to circadian oscillators
Abstract
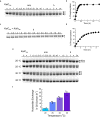
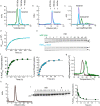
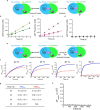
Circadian rhythms play an essential part in many biological processes, and only three prokaryotic proteins are required to constitute a true post-translational circadian oscillator1. The evolutionary history of the three Kai proteins indicates that KaiC is the oldest member and a central component of the clock2. Subsequent additions of KaiB and KaiA regulate the phosphorylation state of KaiC for time synchronization. The canonical KaiABC system in cyanobacteria is well understood3-6, but little is known about more ancient systems that only possess KaiBC. However, there are reports that they might exhibit a basic, hourglass-like timekeeping mechanism7-9. Here we investigate the primordial circadian clock in Rhodobacter sphaeroides, which contains only KaiBC, to elucidate its inner workings despite missing KaiA. Using a combination of X-ray crystallography and cryogenic electron microscopy, we find a new dodecameric fold for KaiC, in which two hexamers are held together by a coiled-coil bundle of 12 helices. This interaction is formed by the carboxy-terminal extension of KaiC and serves as an ancient regulatory moiety that is later superseded by KaiA. A coiled-coil register shift between daytime and night-time conformations is connected to phosphorylation sites through a long-range allosteric network that spans over 140 Å. Our kinetic data identify the difference in the ATP-to-ADP ratio between day and night as the environmental cue that drives the clock. They also unravel mechanistic details that shed light on the evolution of self-sustained oscillators.
© 2023. The Author(s).
Conflict of interest statement
D.K. is co-founder of Relay Therapeutics and MOMA Therapeutics. The remaining authors declare no competing interests.
Figures






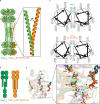



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

