CFTR function, pathology and pharmacology at single-molecule resolution
- PMID: 36949202
- PMCID: PMC10115640
- DOI: 10.1038/s41586-023-05854-7
CFTR function, pathology and pharmacology at single-molecule resolution
Erratum in
-
Author Correction: CFTR function, pathology and pharmacology at single-molecule resolution.Nature. 2023 May;617(7961):E11. doi: 10.1038/s41586-023-06115-3. Nature. 2023. PMID: 37142783 Free PMC article. No abstract available.
Abstract
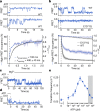
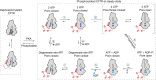
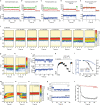
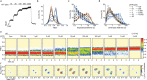
The cystic fibrosis transmembrane conductance regulator (CFTR) is an anion channel that regulates salt and fluid homeostasis across epithelial membranes1. Alterations in CFTR cause cystic fibrosis, a fatal disease without a cure2,3. Electrophysiological properties of CFTR have been analysed for decades4-6. The structure of CFTR, determined in two globally distinct conformations, underscores its evolutionary relationship with other ATP-binding cassette transporters. However, direct correlations between the essential functions of CFTR and extant structures are lacking at present. Here we combine ensemble functional measurements, single-molecule fluorescence resonance energy transfer, electrophysiology and kinetic simulations to show that the two nucleotide-binding domains (NBDs) of human CFTR dimerize before channel opening. CFTR exhibits an allosteric gating mechanism in which conformational changes within the NBD-dimerized channel, governed by ATP hydrolysis, regulate chloride conductance. The potentiators ivacaftor and GLPG1837 enhance channel activity by increasing pore opening while NBDs are dimerized. Disease-causing substitutions proximal (G551D) or distal (L927P) to the ATPase site both reduce the efficiency of NBD dimerization. These findings collectively enable the framing of a gating mechanism that informs on the search for more efficacious clinical therapies.
© 2023. The Author(s).
Conflict of interest statement
S.C.B. has an equity interest in Lumidyne Technologies. The other authors declare no competing interests.
Figures










References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

