A multicentre, patient- and assessor-blinded, non-inferiority, randomised and controlled phase II trial to compare standard and torque teno virus-guided immunosuppression in kidney transplant recipients in the first year after transplantation: TTVguideIT
- PMID: 36949445
- PMCID: PMC10032258
- DOI: 10.1186/s13063-023-07216-0
A multicentre, patient- and assessor-blinded, non-inferiority, randomised and controlled phase II trial to compare standard and torque teno virus-guided immunosuppression in kidney transplant recipients in the first year after transplantation: TTVguideIT
Abstract
Background: Immunosuppression after kidney transplantation is mainly guided via plasma tacrolimus trough level, which cannot sufficiently predict allograft rejection and infection. The plasma load of the non-pathogenic and highly prevalent torque teno virus (TTV) is associated with the immunosuppression of its host. Non-interventional studies suggest the use of TTV load to predict allograft rejection and infection. The primary objective of the current trial is to demonstrate the safety, tolerability and preliminary efficacy of TTV-guided immunosuppression.
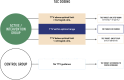
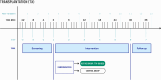
Methods: For this purpose, a randomised, controlled, interventional, two-arm, non-inferiority, patient- and assessor-blinded, investigator-driven phase II trial was designed. A total of 260 stable, low-immunological-risk adult recipients of a kidney graft with tacrolimus-based immunosuppression and TTV infection after month 3 post-transplantation will be recruited in 13 academic centres in six European countries. Subjects will be randomised in a 1:1 ratio (allocation concealment) to receive tacrolimus either guided by TTV load or according to the local centre standard for 9 months. The primary composite endpoint includes the occurrence of infections, biopsy-proven allograft rejection, graft loss, or death. The main secondary endpoints include estimated glomerular filtration rate, graft rejection detected by protocol biopsy at month 12 post-transplantation (including molecular microscopy), development of de novo donor-specific antibodies, health-related quality of life, and drug adherence. In parallel, a comprehensive biobank will be established including plasma, serum, urine and whole blood. The date of the first enrolment was August 2022 and the planned end is April 2025.
Discussion: The assessment of individual kidney transplant recipient immune function might enable clinicians to personalise immunosuppression, thereby reducing infection and rejection. Moreover, the trial might act as a proof of principle for TTV-guided immunosuppression and thus pave the way for broader clinical applications, including as guidance for immune modulators or disease-modifying agents.
Trial registration: EU CT-Number: 2022-500024-30-00.
Keywords: Graft rejection; Immunological monitoring; Immunosuppression; Infection; Kidney transplantation; Personalised medicine; Tacrolimus; Torque teno virus.
© 2023. The Author(s).
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as a potential competing interest concerning the present work: Fanny Gelas, and Philippe Bourgeois are employees of bioMérieux.
Figures

References
-
- Brunet M, van Gelder T, Asberg A, Haufroid V, Hesselink DA, Langman L, et al. Therapeutic Drug Monitoring of Tacrolimus-Personalized Therapy: Second Consensus Report. Ther Drug Monit. 2019;41(3):261–307. - PubMed
-
- Bouamar R, Shuker N, Hesselink DA, Weimar W, Ekberg H, Kaplan B, et al. Tacrolimus predose concentrations do not predict the risk of acute rejection after renal transplantation: a pooled analysis from three randomized-controlled clinical trials(dagger). Am J Transplant. 2013;13(5):1253–61. - PubMed
-
- Anglicheau D, Naesens M, Essig M, Gwinner W, Marquet P. Establishing biomarkers in transplant medicine: a critical review of current approaches. Transplantation. 2016;100(10):2024–38. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

