Localized microglia dysregulation impairs central nervous system myelination in development
- PMID: 36949514
- PMCID: PMC10035254
- DOI: 10.1186/s40478-023-01543-8
Localized microglia dysregulation impairs central nervous system myelination in development
Abstract
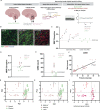
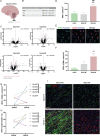
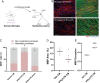
Myelination of neuronal axons is a critical aspect of central nervous system development and function. However, the fundamental cellular and molecular mechanisms influencing human developmental myelination and its failure are not fully understood. Here, we used digital spatial transcriptomics of a rare bank of human developing white matter to uncover that a localized dysregulated innate immune response is associated with impeded myelination. We identified that poorly myelinating areas have a distinct signature of Type II interferon signalling in microglia/macrophages, relative to adjacent myelinating areas. This is associated with a surprising increase in mature oligodendrocytes, which fail to form myelin processes appropriately. We functionally link these findings by showing that conditioned media from interferon-stimulated microglia is sufficient to dysregulate myelin process formation by oligodendrocytes in culture. We identify the Type II interferon inducer, Osteopontin (SPP1), as being upregulated in poorly myelinating brains, indicating a potential biomarker. Our results reveal the importance of microglia-mature oligodendrocyte interaction and interferon signaling in regulating myelination of the developing human brain.
Keywords: Microglia; Myelin; Neuroinflammation; Oligodendrocyte; White matter.
© 2023. The Author(s).
Conflict of interest statement
LY, KT, and YL are employees of Nanostring, Inc.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

