Progression of irradiated mesenchymal stromal cells from early to late senescence: Changes in SASP composition and anti-tumour properties
- PMID: 36949664
- PMCID: PMC10280137
- DOI: 10.1111/cpr.13401
Progression of irradiated mesenchymal stromal cells from early to late senescence: Changes in SASP composition and anti-tumour properties
Abstract
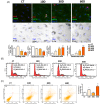
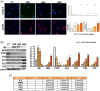
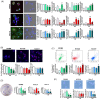
Genotoxic injuries converge on senescence-executive program that promotes production of a senescence-specific secretome (SASP). The study of SASP is particularly intriguing, since through it a senescence process, triggered in a few cells, can spread to many other cells and produce either beneficial or negative consequences for health. We analysed the SASP of quiescent mesenchymal stromal cells (MSCs) following stress induced premature senescence (SIPS) by ionizing radiation exposure. We performed a proteome analysis of SASP content obtained from early and late senescent cells. The bioinformatics studies evidenced that early and late SASPs, besides some common ontologies and signalling pathways, contain specific factors. In spite of these differences, we evidenced that SASPs can block in vitro proliferation of cancer cells and promote senescence/apoptosis. It is possible to imagine that SASP always contains core components that have an anti-tumour activity, the progression from early to late senescence enriches the SASP of factors that may promote SASP tumorigenic activity only by interacting and instructing cells of the immune system. Our results on Caco-2 cancer cells incubated with late SASP in presence of peripheral white blood cells strongly support this hypothesis. We evidenced that quiescent MSCs following SIPS produced SASP that, while progressively changed its composition, preserved the capacity to block cancer growth by inducing senescence and/or apoptosis only in an autonomous manner.
© 2023 The Authors. Cell Proliferation published by European Cell Proliferation Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Tumpel S, Rudolph KL. Quiescence: good and bad of stem cell aging. Trends Cell Biol. 2019;29(8):672‐685. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

