ARIA in patients treated with lecanemab (BAN2401) in a phase 2 study in early Alzheimer's disease
- PMID: 36949897
- PMCID: PMC10026083
- DOI: 10.1002/trc2.12377
ARIA in patients treated with lecanemab (BAN2401) in a phase 2 study in early Alzheimer's disease
Abstract
Introduction: Lecanemab is a humanized immunoglobulin G1 (IgG1) monoclonal antibody that preferentially targets soluble aggregated Aβ species (protofibrils) with activity at amyloid plaques. Amyloid-related imaging abnormalities (ARIA) profiles appear to differ for various anti-amyloid antibodies. Here, we present ARIA data from a large phase 2 lecanemab trial (Study 201) in early Alzheimer's disease.
Methods: Study 201 trial was double-blind, placebo-controlled (core) with an open-label extension (OLE). Observed ARIA events were summarized and modeled via Kaplan-Meier graphs. An exposure response model was developed.
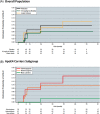
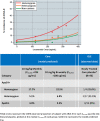
Results: In the phase 2 core and OLE, there was a low incidence of ARIA-E (<10%), with <3% symptomatic cases. ARIA-E was generally asymptomatic, mild-to-moderate in severity, and occurred early (<3 months). ARIA-E was correlated with maximum lecanemab serum concentration and incidence was higher in apolipoprotein E4 (ApoE4) homozygous carriers. ARIA-H and ARIA-E occurred with similar frequency in core and OLE.
Discussion: Lecanemab can be administered without titration with modest incidence of ARIA.
Keywords: ARIA; Alzheimer's disease; anti‐amyloid; exposure response modeling; lecanemab.
© 2023 The Authors. Alzheimer's & Dementia: Translational Research & Clinical Interventions published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Lawrence S. Honig; Research Funding from Abbive, Acumen, Alector, Biogen, Eisai, Genentech, Janssen/J&J, Roche, Transposon, UCB, Vaccinex.; Consulting fees from Alector, Biogen, Cortexyme, Eisai, Medscape, Prevail.; Jerome Barakos; Speaker fees from Biogen. Employee of Clario.; Shobha Dhadda, Michio Kanekiyo, Larisa Reyderman, Michael Irizarry, Lynn D. Kramer, and Chad J. Swanson; Authors are employees of Eisai Inc.; Marwan Noel Sabbagh MD; Ownership interest (Stock or stock options): NeuroTau, uMethod Health, Versanum, Athira, TransDermix, Seq BioMarque, NeuroReserve, Cortexyme/Quince Therapeutics, Lighthouse Therapeutics; Consulting: Alzheon, Biogen, Roche‐Genentech, Eisai, KeifeRx, Lilly, Synaptogenix, NeuroTherapia, T3D, Signant Health, Novo Nordisk; Royalties: Humanix; Board of Director:EIP Pharma. Author disclosures are available in the supporting information.
Figures


References
-
- Rinne JO, Brooks DJ, Rossor MN, et al. 11C‐PiB PET assessment of change in fibrillar amyloid‐beta load in patients with Alzheimer's disease treated with bapineuzumab: a phase 2, double‐blind, placebo‐controlled, ascending‐dose study. Lancet Neurol. 2010;9:363‐372. - PubMed
-
- Ostrowitzki S, Deptula D, Thurfjell L, et al. Mechanism of amyloid removal in patients with Alzheimer disease treated with gantenerumab. Arch Neurol. 2012;69(2):198‐207. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
