Changes of tumor microenvironment in non-small cell lung cancer after TKI treatments
- PMID: 36949948
- PMCID: PMC10025329
- DOI: 10.3389/fimmu.2023.1094764
Changes of tumor microenvironment in non-small cell lung cancer after TKI treatments
Abstract
Non-small cell lung cancer (NSCLC) is the most common lung cancer diagnosis, among which epidermal growth factor receptor (EGFR), Kirsten rat sarcoma (KRAS), and anaplastic lymphoma kinase (ALK) mutations are the common genetic drivers. Their relative tyrosine kinase inhibitors (TKIs) have shown a better response for oncogene-driven NSCLC than chemotherapy. However, the development of resistance is inevitable following the treatments, which need a new strategy urgently. Although immunotherapy, a hot topic for cancer therapy, has shown an excellent response for other cancers, few responses for oncogene-driven NSCLC have been presented from the existing evidence, including clinical studies. Recently, the tumor microenvironment (TME) is increasingly thought to be a key parameter for the efficacy of cancer treatment such as targeted therapy or immunotherapy, while evidence has also shown that the TME could be affected by multi-factors, such as TKIs. Here, we discuss changes in the TME in NSCLC after TKI treatments, especially for EGFR-TKIs, to offer information for a new therapy of oncogene-driven NSCLC.
Keywords: ICIs - immune checkpoint inhibitors; driver mutation; non-small cell lung cancer; tumor microenvironment; tyrosine kinase inhibitor.
Copyright © 2023 Chen, Tang, Liu, Li, Yan, Shangguan, Yang and Liao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
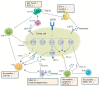
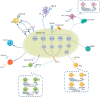
References
-
- Herbst R, Baas P, Kim D, Felip E, Pérez-Gracia J, Han J, et al. . Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet (London England) (2016) 387:1540–50. doi: 10.1016/S0140-6736(15)01281-7 - DOI - PubMed
-
- Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. . Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet (London England) (2017) 389:255–65. doi: 10.1016/S0140-6736(16)32517-X - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

